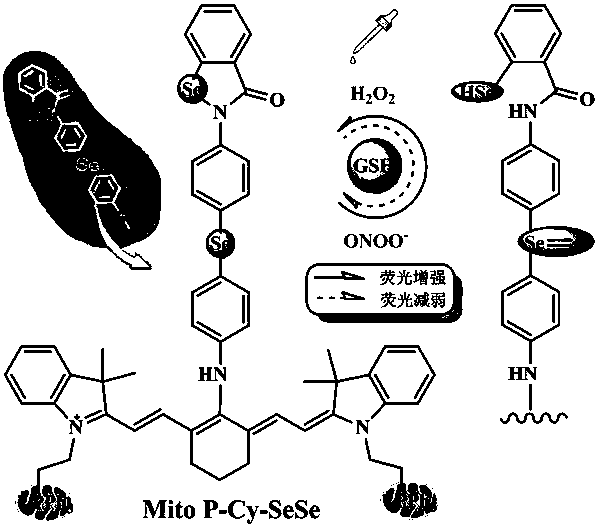
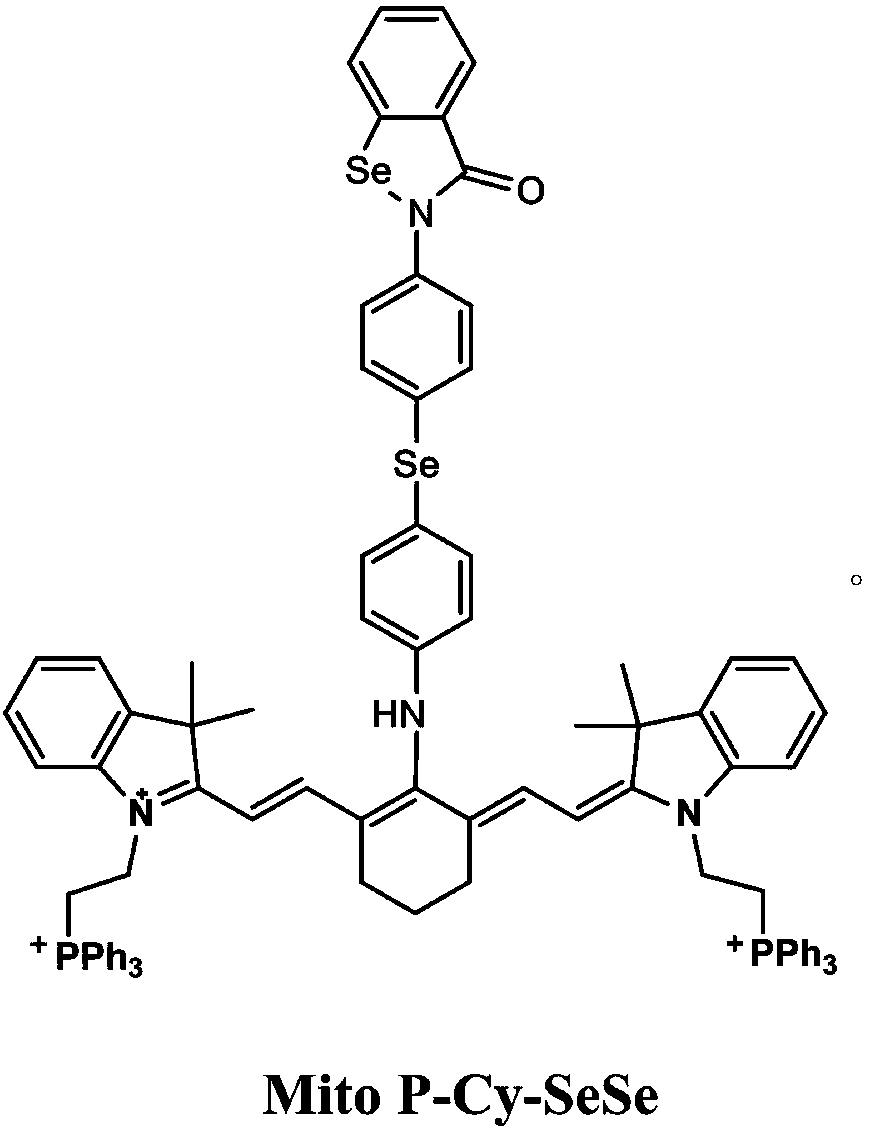
Near infrared fluorescence probe capable of simultaneously detecting hydrogen peroxide and peroxynitrite anoin as well as synthetic method and application thereof
A technology of peroxynitroso group and fluorescent probe is applied in the field of biological detection technology and clinical medical detection, which can solve the problem that the specific recognition of peroxidative free radicals cannot be realized, the probe lacks mitochondrial targeting functional groups, and cannot reflect quantitative changes. Regularity and other issues, to achieve the effect of being conducive to live detection, low equipment investment and production and operation costs, and improved selectivity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The synthetic method of the near-infrared fluorescent probe that simultaneously detects hydrogen peroxide and peroxynitroso anion as described above, the synthetic method comprises the steps:
[0048] Step 1, commercially available p-iodoaniline (220g, 1mol), add selenium powder (40g, 0.5mol), Cu 2 O (1.4g, 0.01mol), ethylenediamine (0.6g, 0.01mol), KOH (12g, 0.01mol), DMSO (500mL), microwave reaction at 100℃, 1h. After the reaction was completed, it was poured into 5 L of ice water, stirred, filtered, washed with a large amount of water, and dried to obtain di-p-amino-phenylselenide with a yield of 72%.
[0049] Step 2, place the two-p-aminophenylselenide prepared in step 1 in a 1500ml three-necked bottle, add 200ml water and 200ml ether, K 2 CO 3 50g. Slowly add 200ml of ether solution of 105g (0.5mol) of compound 2-chloroselenoylbenzoyl chloride dropwise under ice-cooling condition, a milky yellow precipitate is formed, then stir at -5°C for about 2h, then filter ...
Embodiment 2
[0054] The synthetic method of the near-infrared fluorescent probe that simultaneously detects hydrogen peroxide and peroxynitroso anion as described above, the synthetic method comprises the steps:
[0055] Step 1, commercially available p-iodoaniline (220g, 1mol), add selenium powder (80g, 1.05mol), Cu 2 O (7g, 0.05mol), ethylenediamine (3g, 0.05mol), KOH (30g, 0.05mol), DMSO (1000mL), reflux at 180°C for 3h. After the reaction was completed, it was poured into 10L of ice water, stirred, filtered, washed with a large amount of water, and dried to obtain di-p-amino-phenylselenide with a yield of 86%.
[0056] Step 2, place the two-p-aminophenylselenide prepared in step 1 in a 5000ml three-necked bottle, add 500ml water and 500ml ether, K 2 CO 3 500g. Slowly add 210g (1.05mol) of compound 2-chloroselenoylbenzoyl chloride in 500ml of diethyl ether solution under ice-cooling condition, a milky yellow precipitate is formed, then stir at 35°C for about 12h, then filter with suc...
Embodiment 3
[0061] The synthetic method of the near-infrared fluorescent probe that simultaneously detects hydrogen peroxide and peroxynitroso anion as described above, the synthetic method comprises the steps:
[0062] Step 1, commercially available p-iodoaniline (220g, 1mol), add selenium powder (40g, 0.5mol), Cu 2 O (7g, 0.05mol), ethylenediamine (3g, 0.05mol), KOH (30g, 0.05mol), DMSO (1000mL), microwave reaction at 120°C for 1h. After the reaction, pour into 10L of ice water, stir, filter, wash the solid with a large amount of water, and dry to obtain di-p-amino-phenylselenide with a yield of 90%.
[0063]Step 2, place the two-p-aminophenylselenide prepared in step 1 in a 3000ml three-necked bottle, add 500ml acetonitrile, K 2 CO 3 300g. Slowly add 300ml of ether solution of 165g (0.75mol) of compound 2-chloroselenoylbenzoyl chloride dropwise under ice-cooling condition, a milky yellow precipitate is formed, then stir at 15°C for about 6h, then suction filter, take the filter resi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com