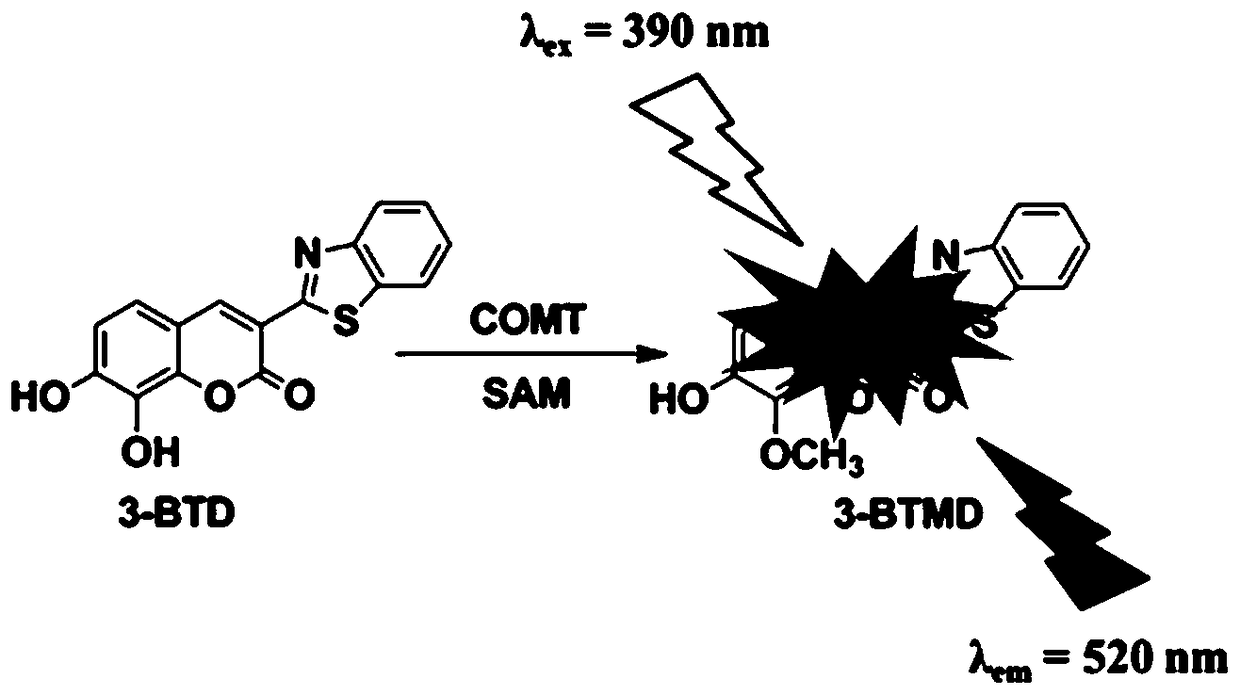
Method for detecting activity of catechol-oxygen-methyltransferase in blood
A technology of methyltransferase and catechol, which is applied in the field of biomedicine, can solve the problems of poor chemical stability and achieve the effects of stable properties, good fluorescence emission spectrum characteristics, good reliability and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
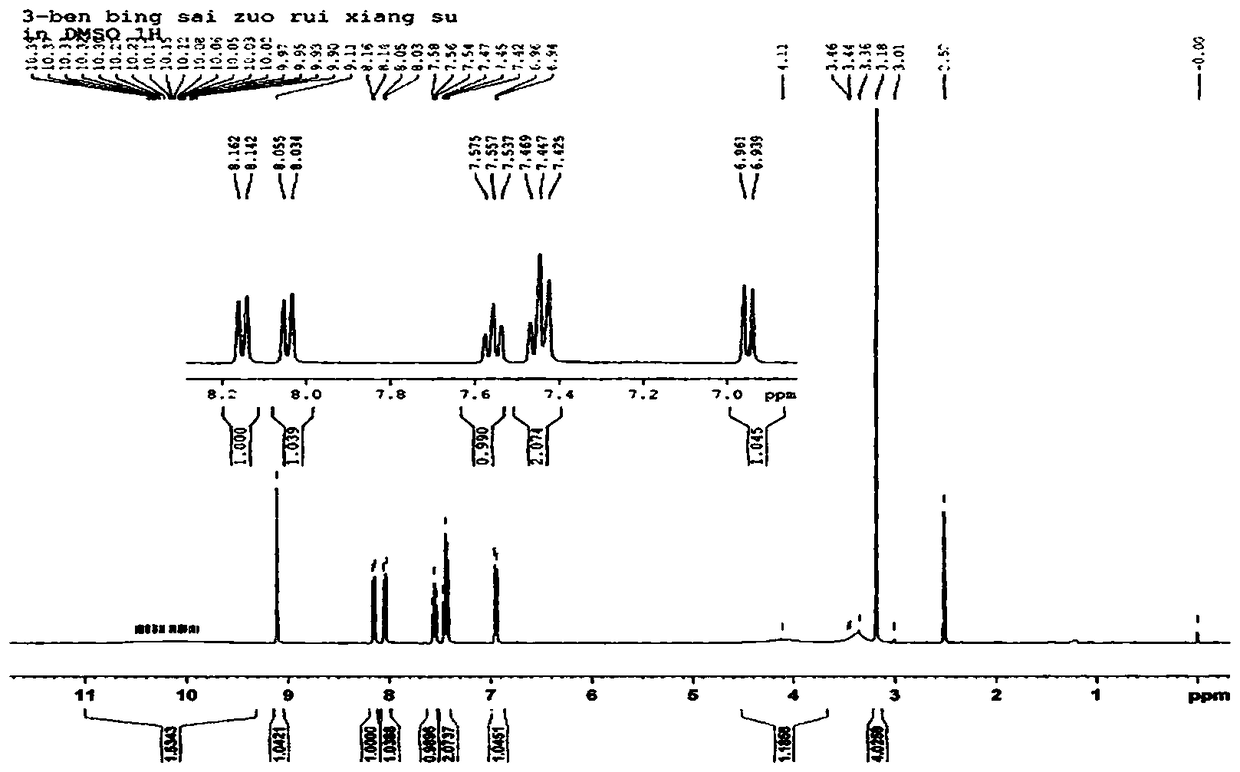
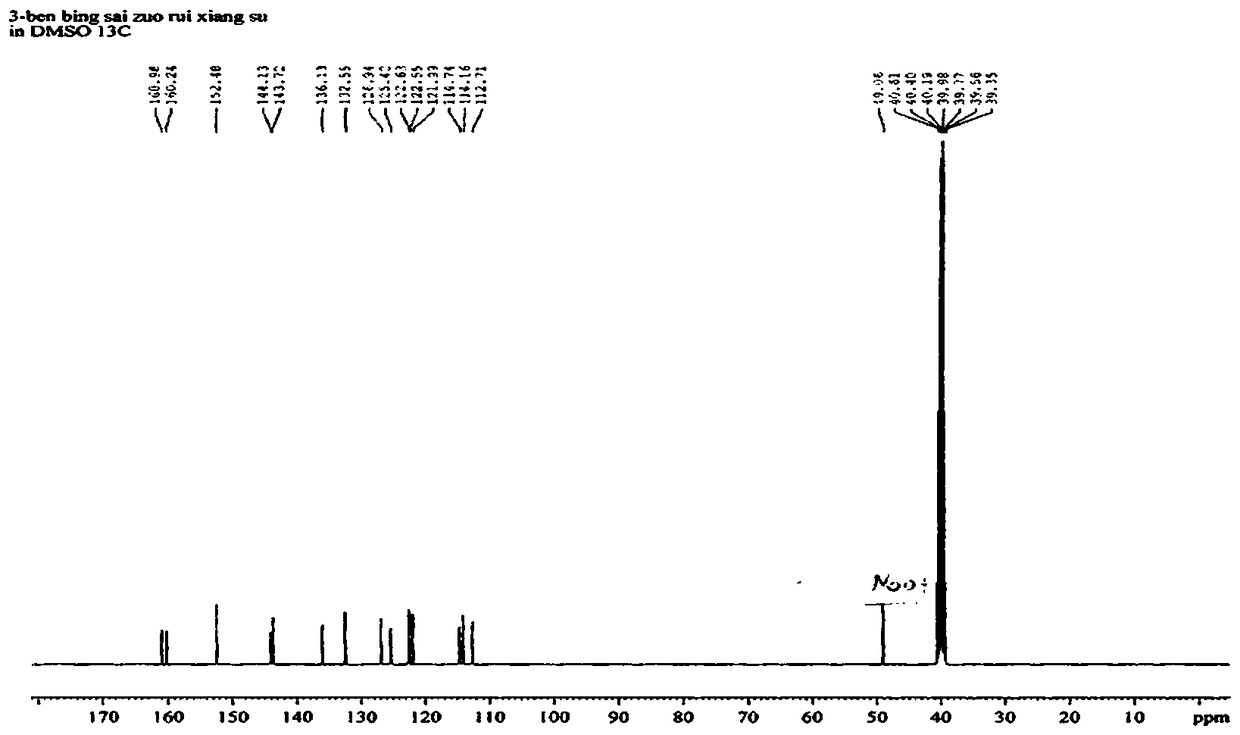
[0030] Synthesis of 3-benzothiazole-7,8-dihydroxycoumarin: Weigh 1.0 g of 2,3,4-trihydroxybenzaldehyde and dissolve it in 25 mL of methanol, add 2-(2-benzothiazole) ethyl acetate Ester 1.5 mL, piperidine 0.1 mL, stirred at room temperature for 20 minutes, heated to 65 °C, reacted for 4 h, TLC detected the end of the reaction. After cooling the reaction solution, add 10 mL of water, filter, and collect the filter cake, which is the crude product. The crude product was added to 20 mL of acetonitrile and refluxed for 1 h, filtered after cooling, and dried in vacuo to obtain 1.8 g of the product 3-benzothiazole-7,8-dihydroxycoumarin as a brownish-yellow solid with a yield of 88%. 1 H NMR spectrum and 13 C NMR spectrum as figure 2 and image 3 shown;
[0031] 1 H NMR (400 MHz, DMSO- d 6 ) δ: 10.59 (s, 1H), 9.68 (s, 1H), 9.11 (s, 1H), 8.16 (d, J= 7.5 Hz, 1H), 8.05 (d, J = 8.0 Hz, 1H), 7.56 (td, J =7.2Hz, J =1.2Hz, 1H), 7.45 (td, J =8.0Hz, J =0.8 Hz, 1H), 7.44 (d, J ...
Embodiment 2
[0035] Determination of fluorescence absorption and emission spectra of substrates and products: Accurately weigh 3-benzothiazole-7,8-dihydroxycoumarin and dissolve in chromatographic grade dimethyl sulfoxide solution to prepare a 50 mmol / L standard The mother liquor was then diluted with 0.5% formic acid in acetonitrile solution and deionized water (1:1) to obtain a standard solution with a final concentration of 1.0 mmol / L, and its fluorescence absorption and emission spectra were measured using a Synergy H1 microplate reader. 3-Benzothiazole-7-hydroxy-8-methoxycoumarin was prepared into a standard solution of the same concentration by the same method, and its fluorescence absorption and emission spectra were measured by using a Synergy H1 microplate reader. The fluorescence absorption and emission spectra of 3-benzothiazole-7,8-dihydroxycoumarin and 3-benzothiazole-7-hydroxy-8-methoxycoumarin are attached Figure 4 with attached Figure 5 shown.
Embodiment 3
[0037] Establishment of high performance liquid chromatography-fluorescence detection and analysis conditions: liquid phase system Shimadzu 20A XR system, equipped with system controller CBM-20A, two LC-20AD pumps, CTO-20A column thermostat, automatic sampler SIL- 20ACHT, one DGU-20A3 vacuum degasser, one RF-20A xs fluorescence detector. The analytical column was a Hedera C18 reverse-phase chromatographic column (150.0 mm×2.1 mm, 3 μm), and the temperature of the column oven was set at 40°C. Mobile phase A was acetonitrile, B was water (containing 0.2% formic acid), and the flow rate was 0.4 ml / min. The gradient elution conditions are: 0-10.0 min, 90% B-5% B; 10.0-13.0 min, 5% B; 13.0-16.0 min, equilibrated to 90% B. The fluorescence detection conditions are: the excitation wavelength is 390 nm, and the emission wavelength is 520 nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com