Cyanine fluorescent dye and synthesis method thereof
A technology for fluorescent dyes and cyanines, applied in the field of organic fluorescent dyes and applications, can solve the problems of difficult to achieve high-sensitivity fluorescence analysis and imaging, short absorption wavelength and emission wavelength, strong fluorescence self-quenching effect, etc., and achieve high yield , high photostability, simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
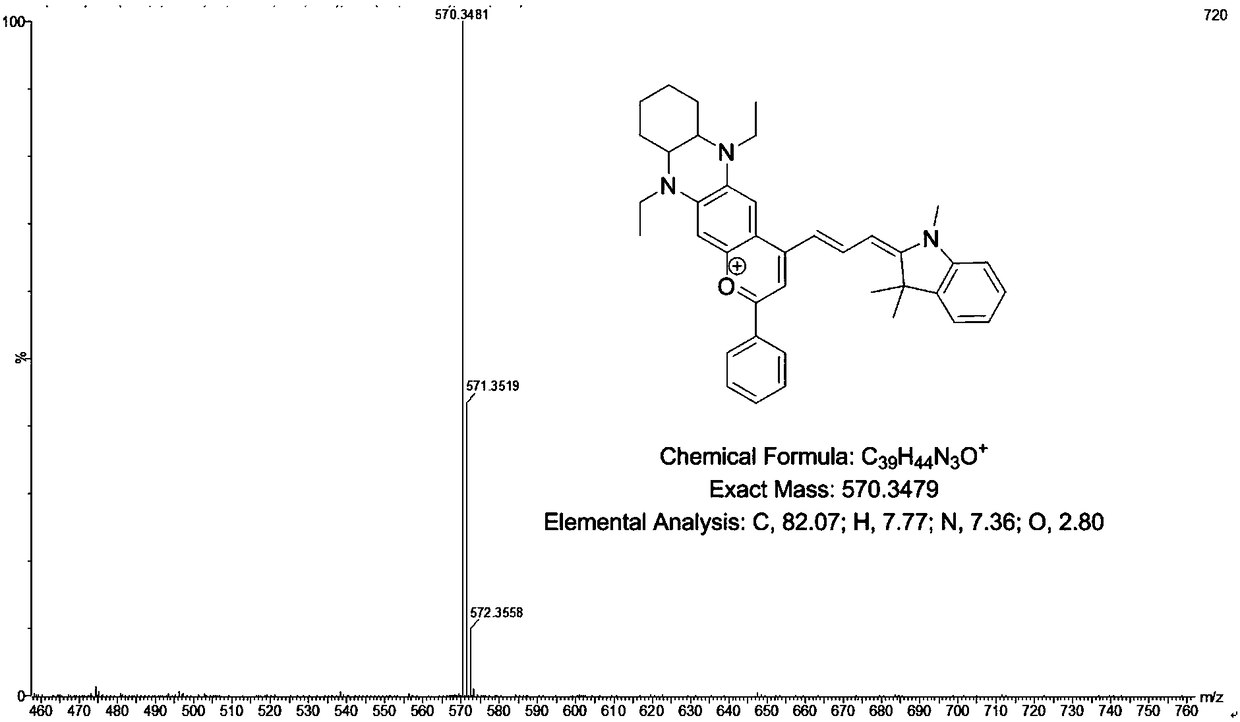
[0046] Embodiment 1: the synthesis of the I formula cyanine fluorescent dye (DQF-692) of n=1
[0047] (1) Synthesis of compound 4 (5,10-diethyl-7-methoxy-1,2,3,4,4a,5,10,10a-swahydrophenazine)
[0048]
[0049] Weigh 4-methoxy o-phenylenediamine (1.3g, 12.0mmol) and 1,2-cyclohexanedione (1.1g, 10.0mmol) and dissolve them in 60mL of ethanol, stir at 60°C for 2h Separation and purification by silica gel column chromatography (the eluent is petroleum ether and dichloromethane, the volume ratio is 1:1), and the light yellow solid compound 3 (7-methoxy-1,2,3,4-tetrahydrophene Dissolve compound 3 (0.9g, 5.0mmol) in 150mL of anhydrous toluene, add sodium borohydride (1.9g, 50.0mmol) in an ice-bath environment, stir for 15min, then slowly add 10mL of glacial acetic acid dropwise for 1h into the reaction solution, after the glacial acetic acid is dripped, maintain the reaction in an ice bath for 30 minutes, then heat the reaction solution to 110 ° C, and reflux for 6 hours. After t...
Embodiment 2
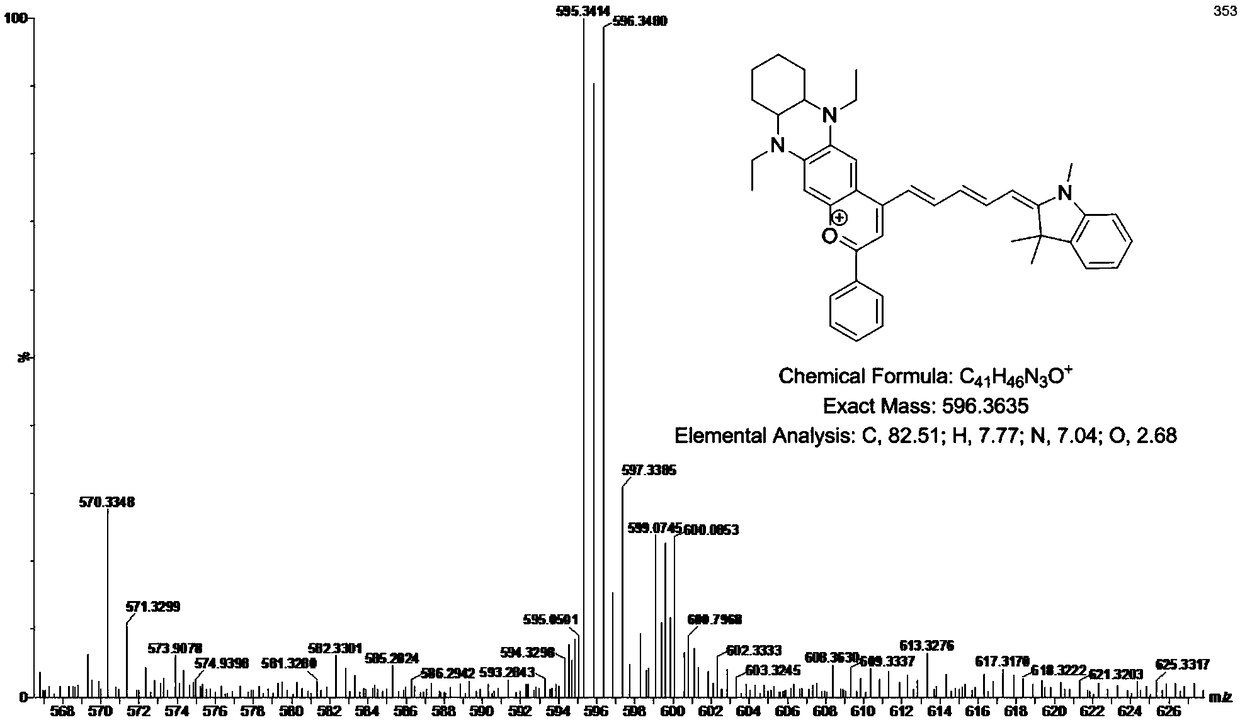
[0056] Embodiment 2: the synthesis of the I formula cyanine fluorescent dye (DQF-780) of n=2
[0057] (1) Intermediate 8 ((1E,2E)-4-((E)-1,3,3-trimethylindoline-2-methylene)-N-phenyl-2-propenyl-1 -imine) synthesis
[0058]
[0059] Dissolve compound 6 (2,3,3-trimethyl-3H-indole) (1.6g, 10mmol) and iodomethane (2.8g, 20mmol) in 20mL of anhydrous acetonitrile, reflux and stir for 6h; After cooling, the separated pink solid obtained compound 7 (1,2,3,3-tetramethyl-3H-indole iodide) after suction filtration, washing and drying; Compound 7 (1,2,3,3 -Tetramethyl-3H-indole iodide) (2.1g, 7mmol) and condensing agent malondialdehyde diphenylamine hydrochloride (2.6g, 10mmol) were dissolved in 30mL n-butanol-toluene mixed solution, at 110°C Reflux reaction for 4h; after the reaction was completed, the solution was concentrated and separated by column chromatography (dichloromethane:ethanol=100:1, v / v) to obtain intermediate 8 ((1E,2E)-4-((E)-1, 3,3-trimethylindoline-2-methylene)-N...
Embodiment 3
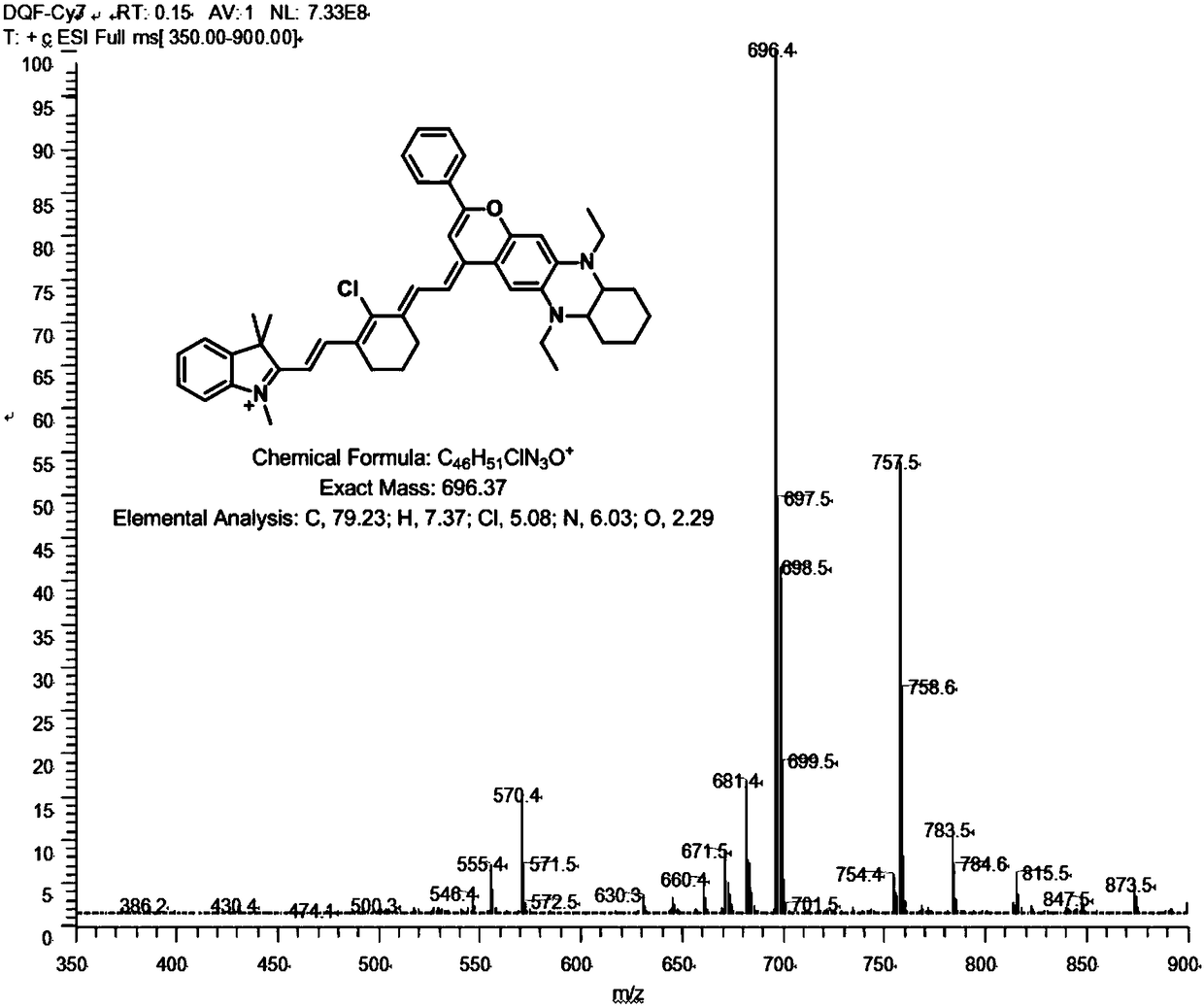
[0063] Embodiment 3: the synthesis of II formula cyanine dye (DQF-Cy7)
[0064] (1) Intermediate 9((E)-2-chloro-3-(2-((E)-1,3,3-trimethylindoline-2-methylene)ethylene)cyclohexyl Synthesis of En-1-ene-1-Carboxaldehyde)
[0065]
[0066]Compound 7 (1,2,3,3-tetramethyl-3H-indole iodide) (3.0 g, 10 mmol) prepared in Example 2 and condensing agent 2-chloro-1-formyl-3-hydroxy Methylenecyclohexene (3.4g, 20mmol) was dissolved in 30mL of n-butanol-toluene mixed solution, reflux reaction at 110°C for 2h; after the reaction was completed, the solution was concentrated and separated by column chromatography (dichloromethane:ethanol=100 :1, v / v) to obtain the intermediate 9 ((E)-2-chloro-3-(2-((E)-1,3,3-trimethylindoline-2-methylene) ethyl) cyclohexene-1-ene-1-carbaldehyde).
[0067] (2) Synthesis of fluorescent dye DQF-Cy7
[0068]
[0069] Anhydrous sodium acetate (2.0g, 25mmol) was mixed with intermediate 9 ((E)-2-chloro-3-(2-((E)-1,3,3-trimethylindoline-2-ylidene Methyl) et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com