Series of micro/nano-rare earth materials and preparation method thereof
A nano-rare earth and rare earth material technology, which is applied in the fields of oxidized rare earth materials, basic rare earth carbonate materials and their preparation, micro/nano rare earth materials and their preparation, oxalic acid rare earth materials, hydroxide rare earth materials, and carbonate rare earth materials. Solve the problems of difficult large-scale production, expensive equipment, slow diffusion speed, etc., and achieve the effects of large-scale production, controllable grain size, and reduced growth rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A. Weigh 37.3g of cerium chloride and dissolve it in 1L of deionized water to prepare a 0.1mol / L cerium chloride solution for use.
[0031] B. Weigh 12.0 g of sodium hydroxide and dissolve it in 1 L of deionized water to prepare a sodium hydroxide solution with a concentration of 0.3 mol / L for use.
[0032] C. Add the solution in step A and step B to the nucleation reactor simultaneously with the same flow rate (100ml / min), control the rotating speed of the nucleation reactor to be 3000 rpm, and form the precipitated slurry from the outlet of the nucleation reactor flow out.
[0033] D. The slurry obtained from the reaction was placed in a crystallization kettle and crystallized at 80° C. for 5 hours, then filtered and washed, and then dried at 60° C. for 12 hours. Cerium hydroxide can be obtained, the chemical formula is Ce(OH) 3 .
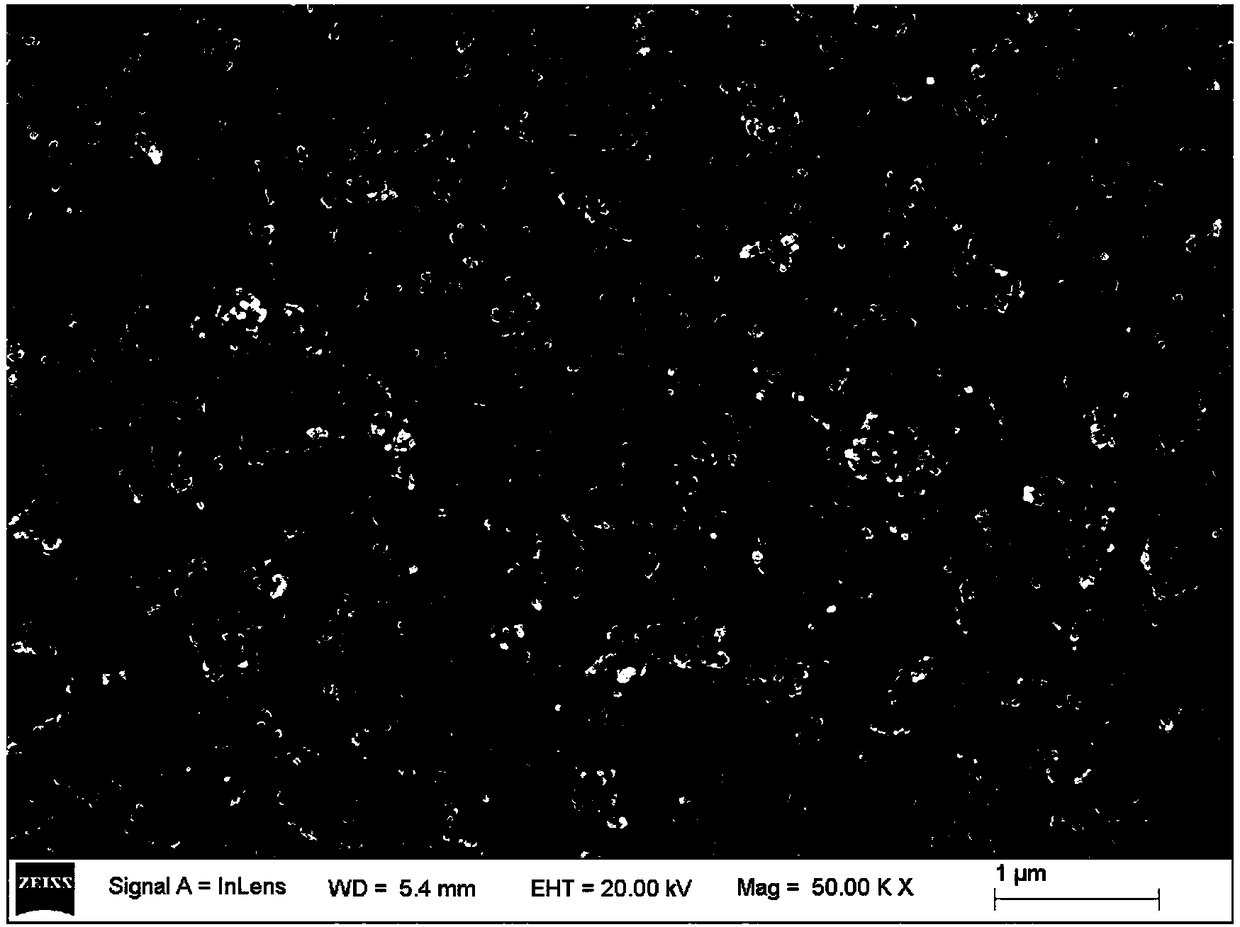
[0034] figure 1 It is the scanning electron microscope picture of the prepared sample, and it can be seen from the picture that the p...
Embodiment 2
[0036] A. Weigh 37.1g of lanthanum chloride and dissolve it in 500mL of deionized water to prepare a 0.2mol / L lanthanum chloride solution for use.
[0037] B. Weigh 12.0g of sodium hydroxide and dissolve it in 500mL of deionized water to prepare a sodium hydroxide solution with a concentration of 0.6mol / L, which is ready for use.
[0038] C. Add the solution in step A and step B to the nucleation reactor simultaneously with the same flow rate (100ml / min), control the rotating speed of the nucleation reactor to be 3000 rpm, and form the precipitated slurry from the outlet of the nucleation reactor flow out.
[0039] D. The slurry obtained from the reaction was placed in a crystallization kettle for crystallization at 80° C. for 5 hours, filtered and washed, and then dried at 60° C. for 12 hours. Lanthanum hydroxide can be obtained, the chemical formula is La(OH) 3 .
[0040] figure 2 is a scanning electron microscope image of the prepared sample, from which it can be seen ...
Embodiment 3
[0042] A. Weigh 37.3g of cerium chloride and dissolve it in 1L of deionized water to prepare a 0.1mol / L cerium chloride solution for use.
[0043] B. Weigh 12.0 g of sodium hydroxide and dissolve it in 1 L of deionized water to prepare a sodium hydroxide solution with a concentration of 0.3 mol / L, which is ready for use.
[0044]C. Add the solution in step A and step B to the nucleation reactor simultaneously with the same flow rate (100ml / min), control the rotating speed of the nucleation reactor to be 3000 rpm, and the precipitated slurry formed from the outlet of the nucleation reactor flow out.
[0045] D. The slurry obtained from the reaction was placed in a crystallization kettle and crystallized at 80° C. for 5 hours, then filtered and washed, and then dried at 60° C. for 12 hours.
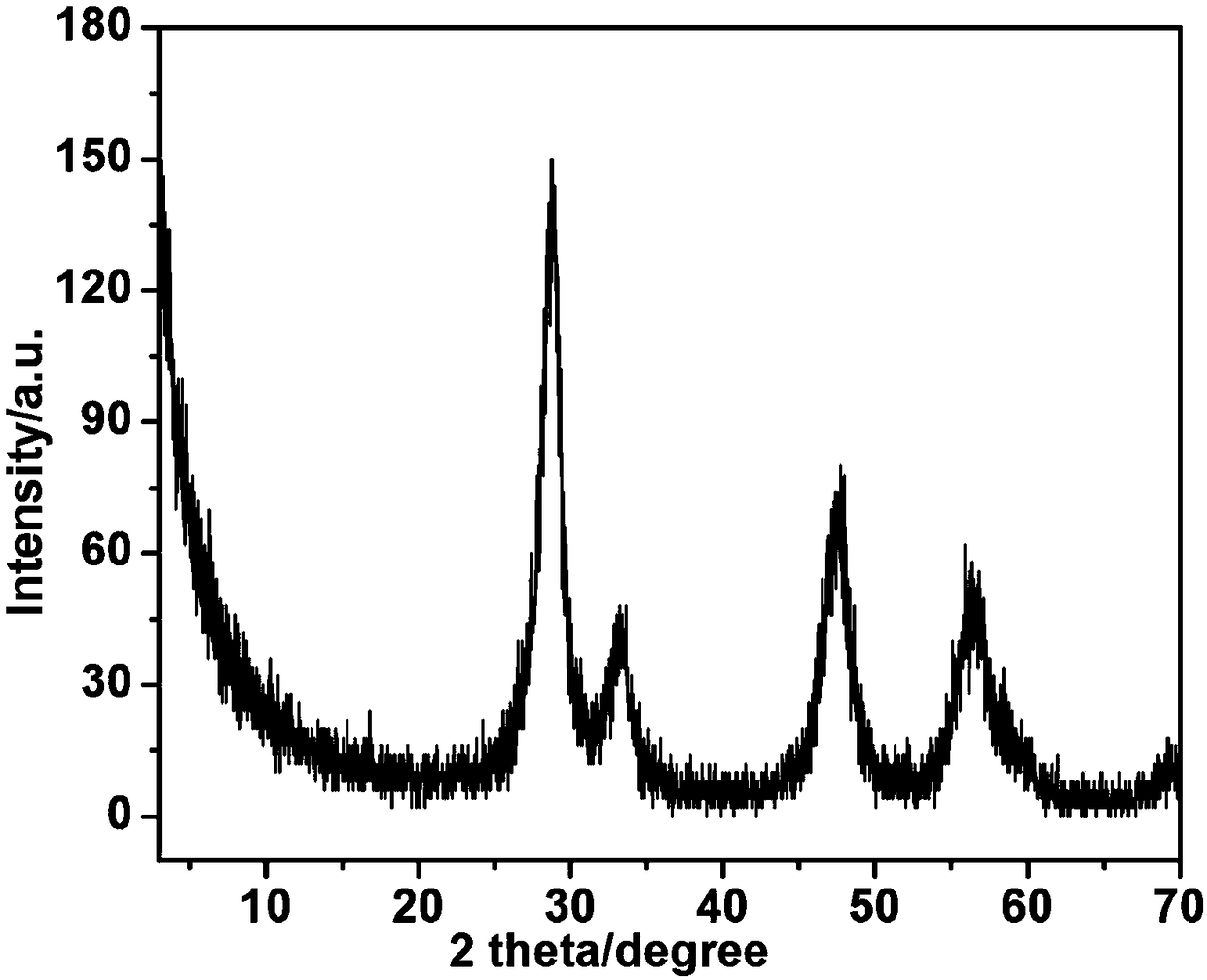
[0046] E. Put the product obtained in step D into a muffle furnace for roasting, keep it warm at 450°C for 3 hours and then lower it to room temperature naturally to obtain cerium oxide, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com