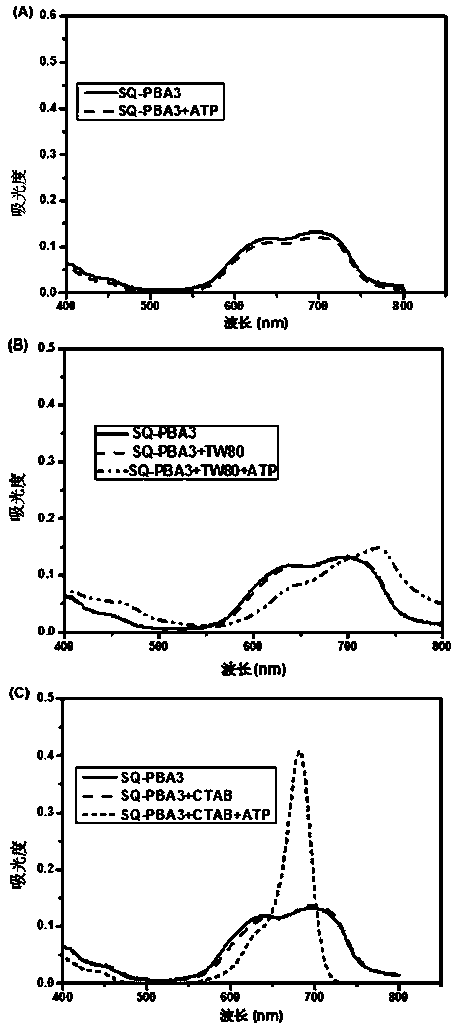
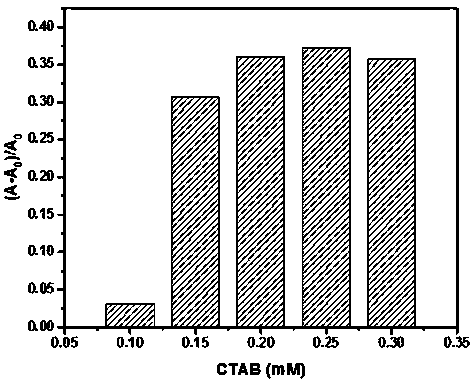
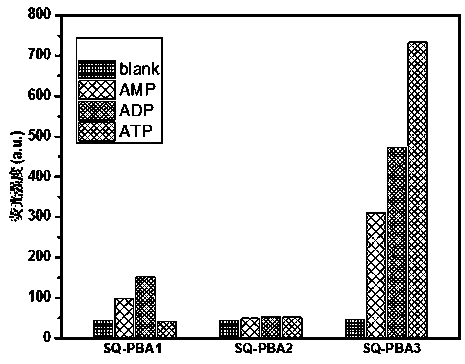
Phenylboronic acid modified near-infrared squaraine dyes as well as preparation method and application thereof
An infrared method and phenylboronic acid technology, applied in the field of analytical chemistry, can solve the problems of inability to realize real-time and in-situ detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of:
[0048] First add 20 mL of ethanol to a 50 mL round-bottomed flask, then add 2.00 g of squaraine (17.6 mmol), and reflux at 80°C for 3 hours. After the squaraine is completely dissolved, remove the ethanol by rotary evaporation under reduced pressure. After repeating this operation three times Reflux reaction again 24 hours, after reaction finishes, adopt sherwood oil: ethyl acetate is 2:1 (v / v) to carry out silica gel column chromatography separation as eluent, obtain light yellow oily liquid product 2.09 g, productive rate is 70 %. 1 H NMR (400 MHz, CDCl 3 ) δ 4.74 (q, J = 7.1Hz, 4H), 1.48 (t, J = 7.1 Hz, 6H).
Embodiment 2
[0050] Dicyanovinyl squaraine derivatives Preparation of:
[0051] Dissolve 1.77 g of malononitrile (26.8 mmol) in 35 mL of dry benzene, place in a 100 mL round-bottomed flask, and drop into 5 g of the (29.4 mmol), then slowly added 2.98 g triethylamine (29.4 mmol) into the above reaction solution, and stirred at room temperature for 6 h. After the reaction was completed, the reaction bottle was placed in the refrigerator to cool for 24 hours, and the 2 Cl 2 After extraction, the organic layer was concentrated in vacuo to obtain 5.28 g of a yellow solid product with a yield of 62%. 1 H NMR (400 MHz, CDCl 3 )δ 9.19 (brs, 1H), 4.76 (q, J = 7.0 Hz, 2H), 3.32-3.25 (m, 6H), 1.99 (s, 1H), 1.49 (t, J = 7.1 Hz, 3H), 1.38 (t, J = 7.3 Hz, 9H).
Embodiment 3
[0053] 2,3,3-Trimethylindole preparation of
[0054] Add 20 mL of absolute ethanol containing 500 mg of phenylhydrazine (4.62 mmol) into a 50 mL round bottom flask, then slowly add 438 mg of methyl isopropyl ketone (5.09 mmol) into the above solution dropwise, the solution becomes light Then 0.25 mL of concentrated sulfuric acid was added dropwise within 0.5 h. At this time, the reaction system turned into a yellow turbid liquid again, and the reaction was refluxed at 80°C for 3 h. As the reaction temperature increased, the color of the reaction system gradually changed from yellow to orange, and then to an orange-red turbid solution. It was detected by TLC that the reaction was complete. After the reaction solution was cooled to room temperature, NaOH solution was added dropwise to adjust the pH to 8. use CH 2 Cl 2 Extracted three times, removed the solvent under reduced pressure, and purified by silica gel column chromatography using petroleum ether:ethyl acetate 3:1 (v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com