Salt preparing method and system
A salt crystal and saturation technology, which is applied in the field of salt production system, can solve the problems of increased cost and increased processing technology, and achieve the effects of reduced cleaning frequency, high purity, and prolonged growth time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
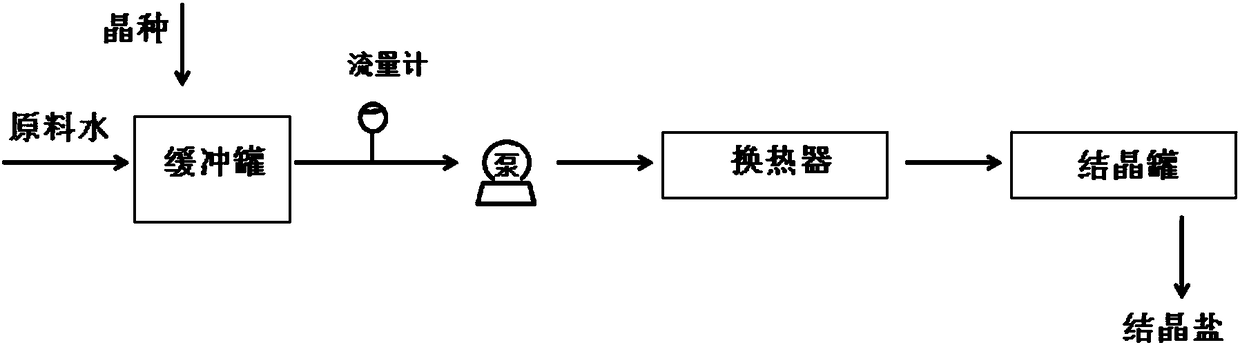
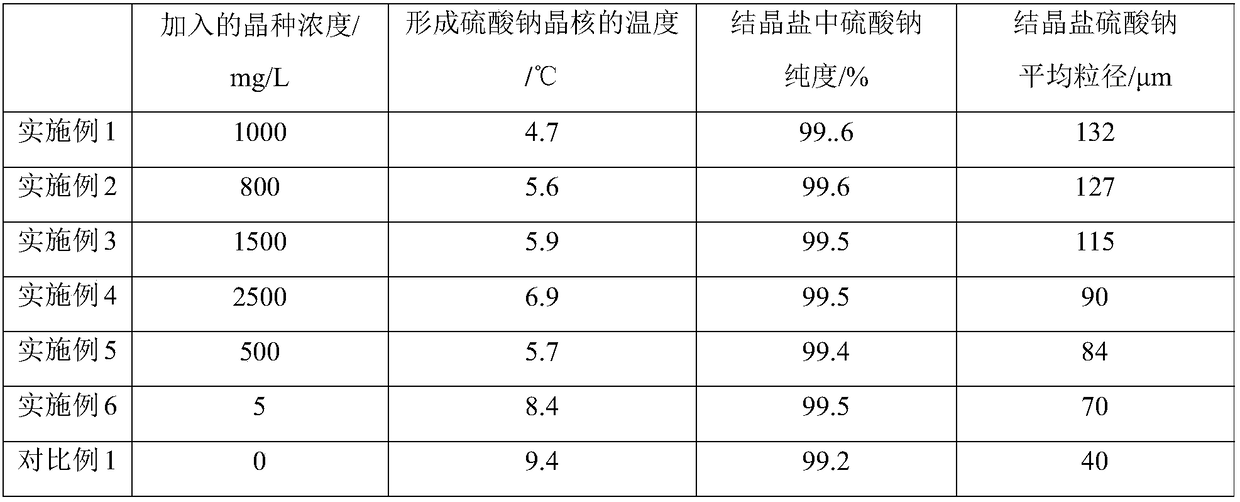
[0055] Synthetic brine-containing simulated raw material water is prepared in a buffer tank, wherein the brine is a mixed solution of sodium sulfate and sodium chloride at 10°C (the temperature of the brine is determined by preparing the brine and the crystallization effluent obtained from the subsequent crystallization separation process) heat exchange treatment to control), the massfraction of sodium sulfate is 8%, and the massfraction of sodium chloride is 2%. According to the Phreeqc simulation, the temperature when the sodium sulfate in the brine is saturated is 10°C, so the brine is a saturated solution of sodium sulfate at this time, and there is no salt precipitation. Add solid sodium sulfate to the brine in a buffer tank to obtain a buffer solution, wherein, based on 1L of buffer solution, the amount of sodium sulfate seed crystals added is 1000mg, and the buffer solution is pumped at a flow rate of 3m / s while stirring. Pour into a coil-type stainless steel heat excha...
Embodiment 2
[0057] Synthetic brine-containing simulated raw material water was prepared in a buffer tank, wherein the brine was a mixed solution of sodium sulfate and sodium chloride at 25°C, the mass fraction of sodium sulfate was 10%, and the mass fraction of sodium chloride was 2%. According to the Phreeqc simulation, there is no salt precipitation in the brine at 25°C. Add the solid-liquid mixed saturated solution of the sodium sulfate crystalline salt that subsequent crystallization separation process obtains to this containing brine in buffer tank, obtain buffer solution, wherein, in 1L buffer solution, the addition amount of sodium sulfate seed crystal is 800mg, while While stirring, pump the buffer solution at a flow rate of 4 m / s into a coiled stainless steel heat exchanger with a pipe diameter of 6 mm for cooling. The refrigerant in the heat exchanger is 20% by weight ethylene glycol solution at 0°C. After the buffer solution is cooled by a heat exchanger, the temperature drops ...
Embodiment 3
[0059] Synthetic brine-containing simulated raw material water was prepared in a buffer tank, wherein the brine was a sodium sulfate solution at 20° C., and the mass fraction of sodium sulfate was 9%. According to the Phreeqc simulation, there is no salt precipitation in the brine at 20°C. Add solid sodium sulfate to the brine in a buffer tank to obtain a buffer solution, wherein, based on 1L of buffer solution, the amount of sodium sulfate seed crystals added is 1500 mg, and the buffer solution is pumped at a flow rate of 5 m / s while stirring. Pour into a coil-type stainless steel heat exchanger with a pipe diameter of 6mm for cooling, and the refrigerant of the heat exchanger is frozen brine at -2°C. After the buffer solution is cooled by a heat exchanger, the temperature drops to 2° C. (at this temperature, the degree of saturation of sodium sulfate is 6% by weight), and the cooled buffer solution enters the crystallization tank for crystallization separation. The residence...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com