Preparation method of acetylsalicylic acid colon positioning bioadhesion type sustained release capsules
A technology for acetylsalicylic acid and colon positioning, which is applied in the direction of microcapsules, capsule delivery, medical preparations of non-active ingredients, etc., to achieve the effect of simple and stable preparation technology, stable performance, and good research and development application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
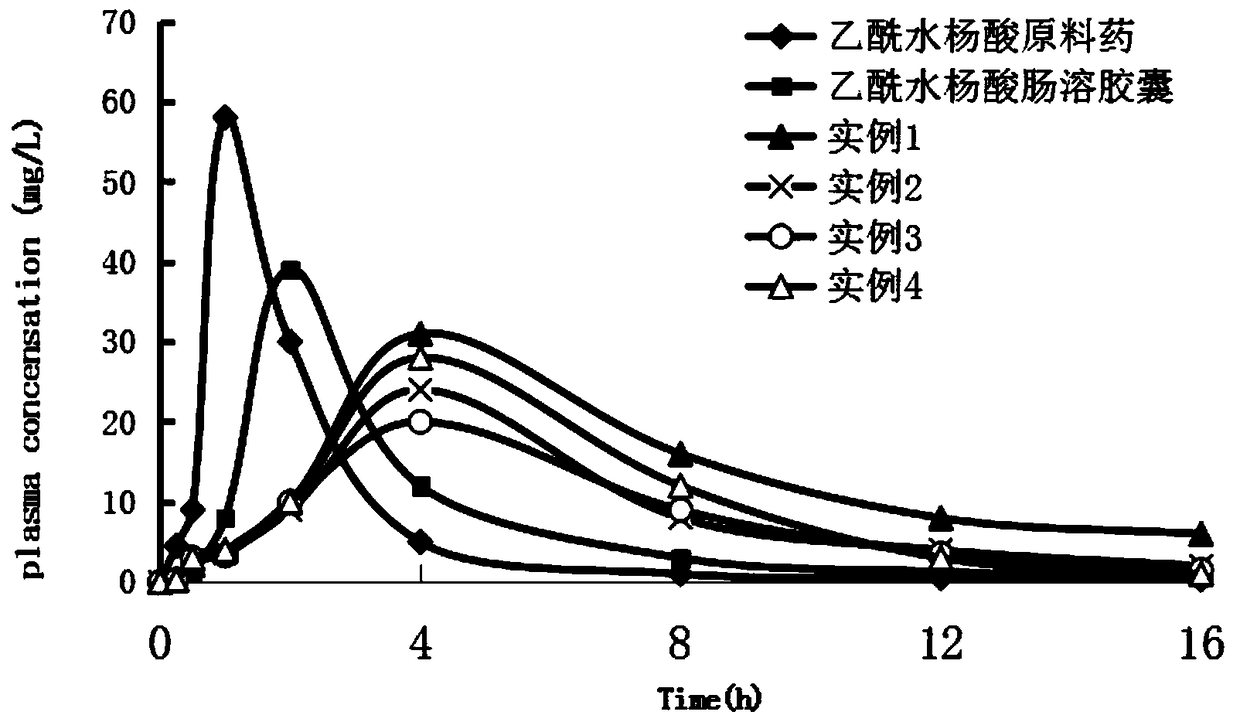
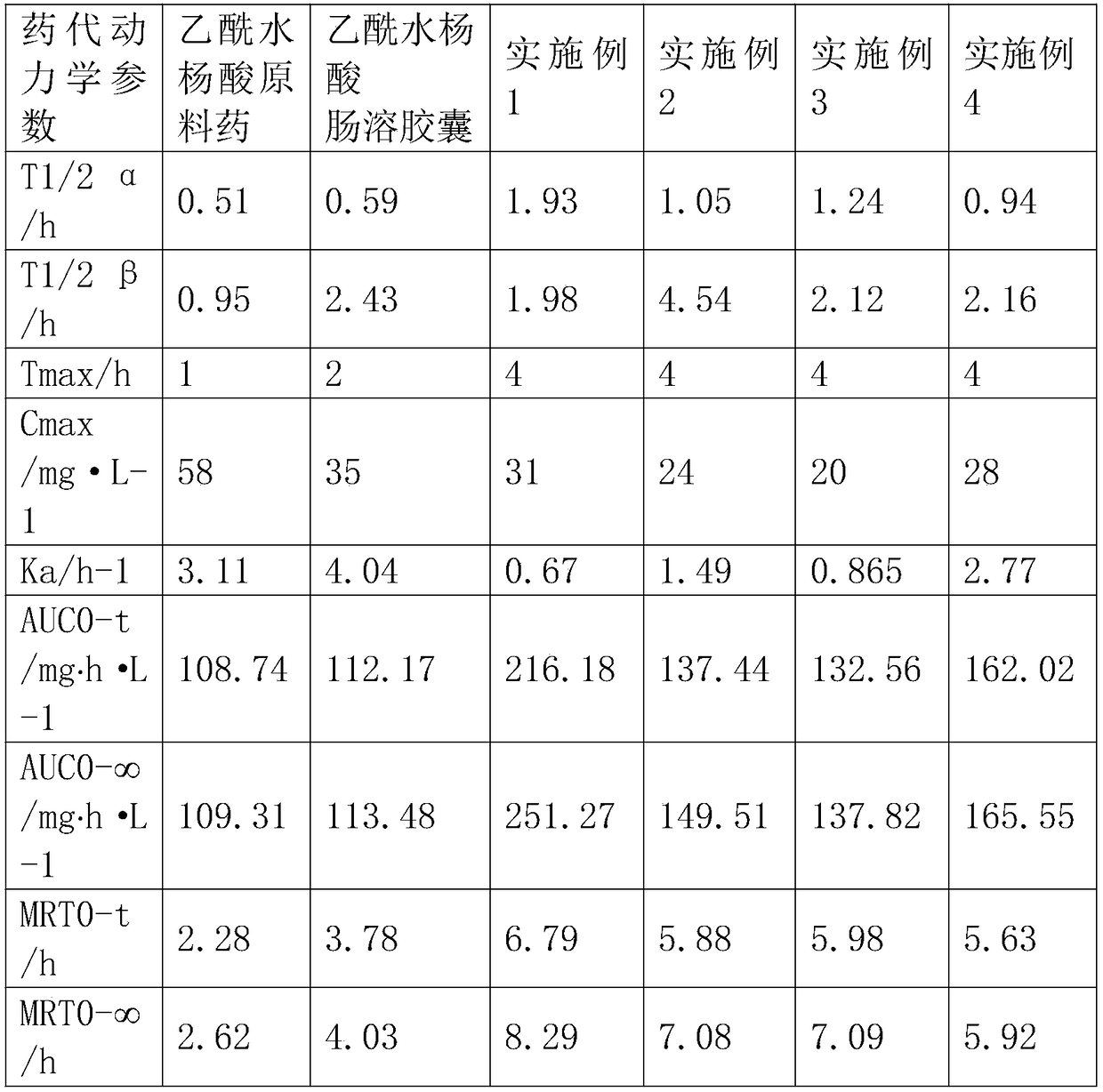
Image
Examples
Embodiment 1
[0031] First fully dissolve β-cyclodextrin and gum arabic respectively, prepare a solution with a concentration of 10% according to a mass ratio of 15:1 between β-cyclodextrin and gum arabic, and mix β-cyclodextrin / gum arabic Glue solution and acetylsalicylic acid crude drug are mixed after being 10:1 by mass ratio, pour into in the dilute hydrochloric acid solution, and add a certain amount of Tween 80 solution wherein, adjust solution pH3, homogeneous pressure is 10MPa, homogeneous The homogenization time is 90s, the homogenization temperature is 45°C, the homogenization is fully emulsified, the vacuum defoaming is 5min, and it is introduced into the spray drying granulator, and the spray drying parameters are adjusted as follows: injection temperature 10°C, injection flow rate 50ml / min, air inlet The temperature is 100°C, and the air outlet temperature is 10°C to obtain acetylsalicylic acid slow-release powder microspheres, which are mixed with hydroxypropyl methylcellulose ...
Embodiment 2
[0033]First fully dissolve the β-cyclodextrin and gelatin respectively, and prepare a solution with a concentration of 15% according to the mass ratio of β-cyclodextrin and gelatin at 1:15, and mix the β-cyclodextrin and gelatin solution with After the acetylsalicylic acid raw material is mixed at a mass ratio of 5:1, it is poured into a deionized aqueous solution, and a certain amount of sodium lauryl sulfate solution is added thereto to adjust the pH to 6, and the homogeneous pressure is 30MPa. The time is 120s, the homogenization temperature is 50°C, the homogenization is fully emulsified, the vacuum defoaming is 8min, and it is introduced into the spray drying granulator, and the spray drying parameters are adjusted as follows: sample injection temperature 20°C, sample flow rate 100ml / min, 150°C and 30°C air outlet temperature to obtain acetylsalicylic acid slow-release powder microspheres, pass through a 100-mesh sieve and mix with hydroxypropyl methylcellulose (HPMC), PVP...
Embodiment 3
[0035] First fully dissolve gum arabic and gelatin respectively, gum arabic and gelatin are made into a solution with a concentration of 8% according to the mass ratio of 3:1, and mix gum arabic and gelatin solution with acetylsalicylic acid raw material drug by mass ratio under stirring condition After mixing 8:1, pour it into the dilute ethanol solution, and add a certain amount of Span solution to it, adjust the pH to 9, the homogenization pressure is 50MPa, the homogenization time is 150s, the homogenization temperature is 40°C, and the homogenization temperature is 40°C. Fully emulsify, vacuum degassing for 10 minutes, import into the spray drying granulator, adjust the spray drying parameters as follows: sample inlet temperature 30°C, sample flow rate 200ml / min, inlet air temperature 200°C, outlet air temperature 60°C, and acetyl water is obtained Salicylic acid slow-release powder microspheres, after passing through 80 mesh sieve, mix with hydroxyethyl cellulose (HEC), c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com