Quinoid compounds and preparation method and applications thereof
A compound and quinone-based technology, which is applied in the preparation of carbon-based compounds, heterocyclic compounds, and organic compounds, can solve the problems of regulating the photophysical properties and carrier transport properties of materials, complex synthesis, and high price. Achieve the effect of improving electron transport performance, strong intermolecular force and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] The synthesis process of compound 1: the compound 9,9-dioctyl-2,7-dibromofluorene (1.5g, 2.73mmol) was dissolved in 40ml tetrahydrofuran, stirred evenly, cooled to -78°C, and then added BuLi dropwise (2.73ml, 6.84mmol, 2.5M), stirred at -78°C for 1h, added DMF (1.69ml, 21.88mmol) dropwise, kept stirring at -78°C for 2h, then raised to room temperature and continued to react for 1h. The reaction solution was poured into water and stirred for 20 min, CH 2 Cl 2 Extraction, washed with water, anhydrous MgSO 4 After drying, filtering, and removing the solvent under reduced pressure, it was separated with a silica gel column (petroleum ether: ethyl acetate = 20:1, volume ratio) to obtain a white solid (1.07 g, yield: 88%).
Embodiment 2
[0034]
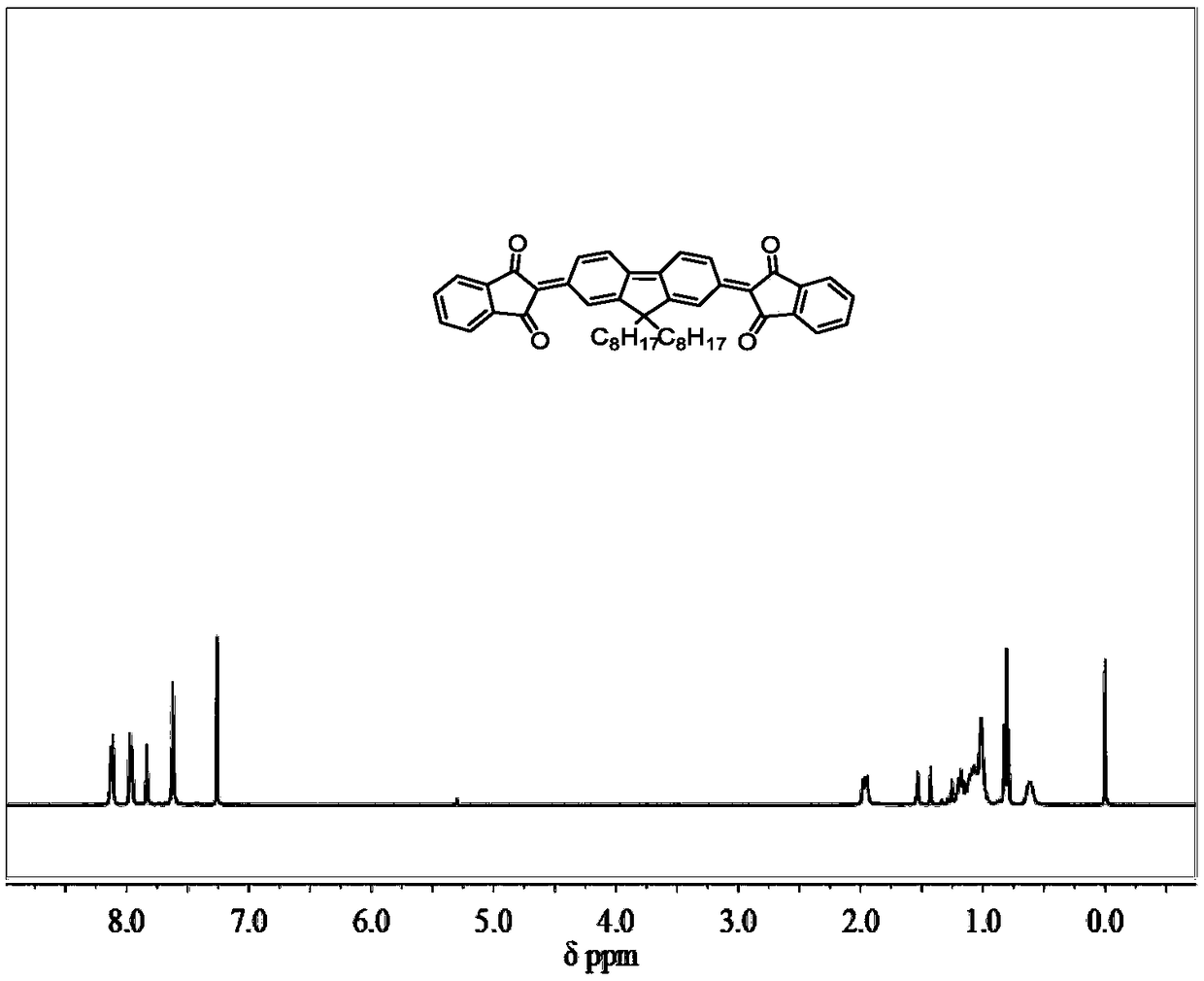
[0035] Synthesis process of compound 2: Compound 1 (1g, 2.24mmol) and phenylpeptide (625mg, 4.70mmol) were dissolved in ethyl propionate 17.2ml, and a methanol solution of sodium methoxide (2.49ml, 5.4M) was added. After reacting at 70°C for 4 hours, the solvent was removed under reduced pressure. After dissolving the residue with water, add 2mol / L hydrochloric acid solution, extract the mixture with chloroform, wash with water, anhydrous MgSO 4 Dry and filter. After the solvent was removed under reduced pressure, it was separated with a silica gel column (eluent: petroleum ether: dichloromethane = 2:3, volume ratio) to obtain a dark red viscous product (1.44 g, yield: 95%). 1 H NMR (CDCl 3 , 400MHz, ppm): δ8.11-8.07(dd, J=4Hz, 4H), 7.94-7.90(dd, J=4Hz, 4H), 7.63-7.57(d, J=8Hz, 2H) 7.21(s, 2H) 7.11-7.05 (d, J = 8Hz, 2H) 4.35-4.31 (s, 2H) 1.93-1.85 (m, 4H) 1.32-0.98 (m, 36H) 0.93-0.82 (t, J = 6, 6H) ), 0.64-0.59 (m, 4H).
Embodiment 3
[0037]
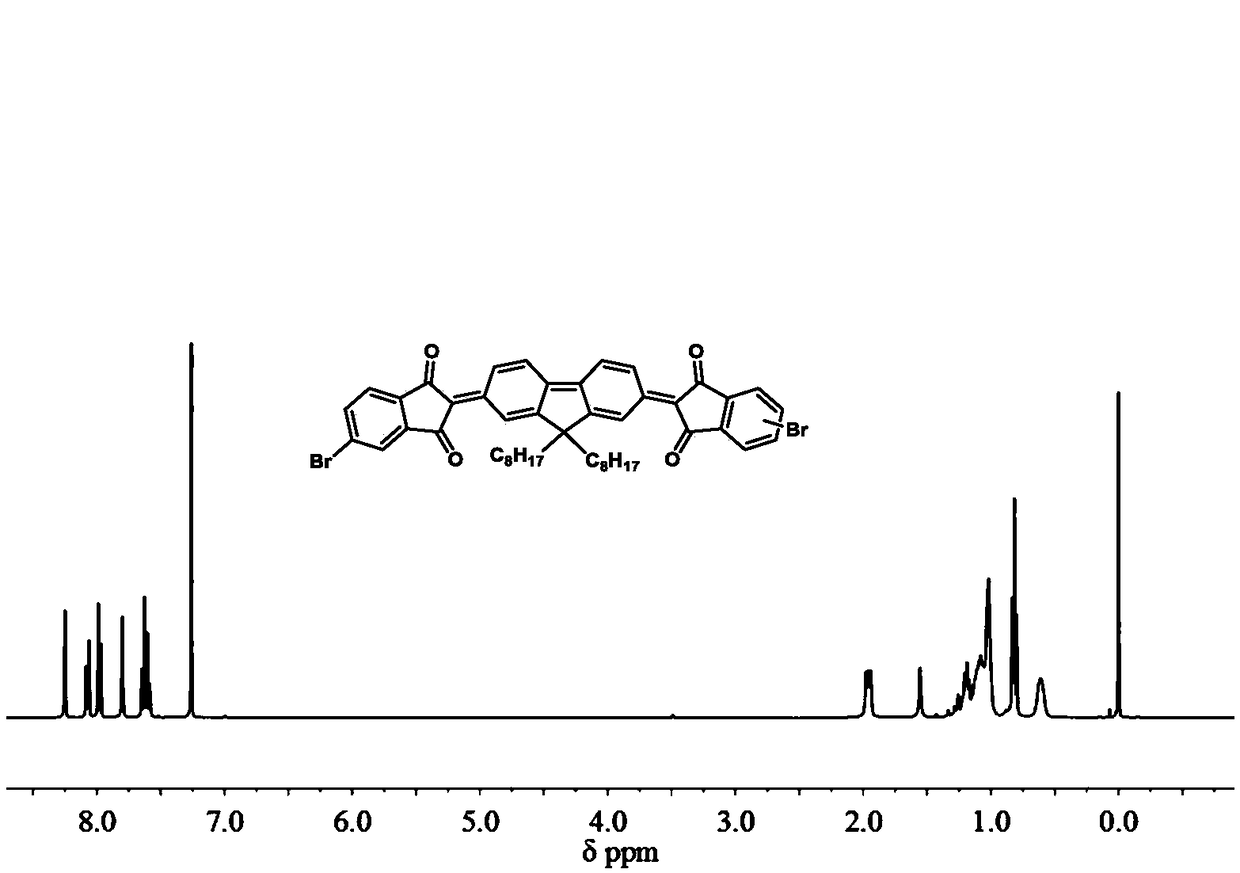
[0038] The synthesis process of compound 3: Dissolve compound 2 (1 g, 1.47 mmol) in 31 ml of acetonitrile, slowly add 31 ml of bromine water dropwise, and react at room temperature for 12 h. Chloroform extraction reaction solution, washed with water, anhydrous MgSO 4 After drying, filtering, and removing the solvent under reduced pressure, it was separated by silica gel column (eluent: petroleum ether: dichloromethane = 1:1, volume ratio) to obtain yellow solid 3 (927 mg, yield: 93%). 1 HNMR (CDCl 3 , 400MHz, ppm): δ8.16-8.10(dd, J=4Hz, 4H), 8.00-7.94(dd, J=4Hz, 4H), 7.86-7.84(s, 2H), 7.65-7.59(s, 4H) 2.02-1.91 (m, 4H) 1.36-0.97 (m, 36H) 0.93-0.82 (t, J = 6, 6H) 0.68-0.57 (m, 4H). The H NMR spectrum of quinone compound 3 is as follows figure 1 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| electron mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com