Light-emitting organic metal silver compound with binary emission as well as preparation and application thereof
A technology of silver complexes and organometallics, which is applied in the preparation and optical properties of organometallic silver halide cluster complexes, can solve the problems of temperature and luminescence sensing staying in the ideal stage, and achieve high actual production repeatability, strong photoinduced Excellent effect of luminescence and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
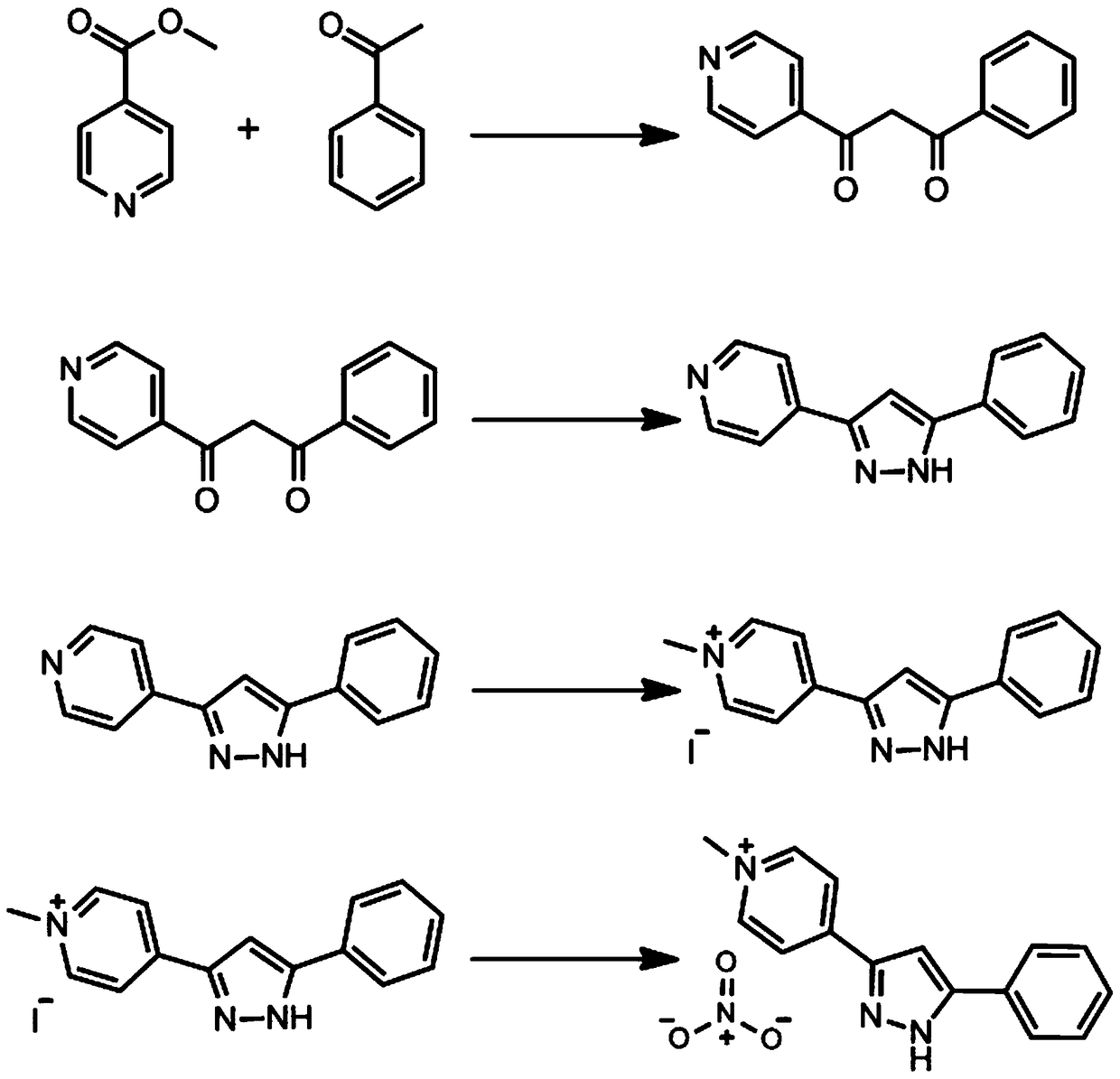
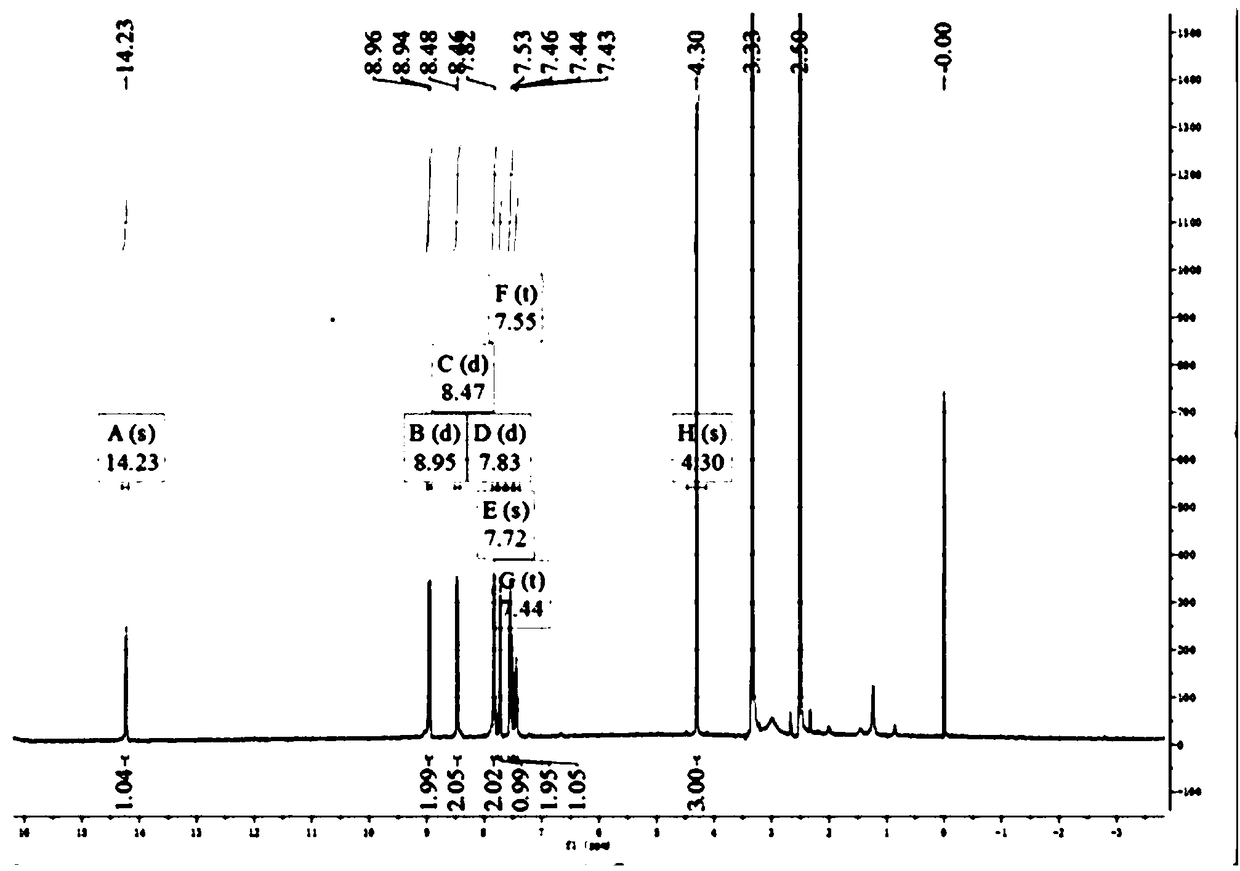
[0059] 1. Organic ligand 1-methyl-4-(3-(5-phenyl-pyrazol) base) pyridinium nitrate (HL-NO 3 ) synthetic route such as figure 1 As shown, it mainly includes the following steps:
[0060] The first step: Potassium tert-butoxide (11.2g, 0.1mol) was dissolved in 200ml tetrahydrofuran, after stirring evenly, add acetophenone (14.0ml, 0.11mol) and methyl 4-picolinate (12.0ml, 0.10mol ), after stirring for 12 hours, 60 mL of an aqueous solution containing 20 mL of acetic acid was added to the substance, and after sufficient stirring, the mixture was extracted with 3 × 20 mL of ether, and the desired product was obtained after the ether was spin-dried in vacuo.
[0061] The second step: the product from the previous step was dissolved in 200 ml of ethanol and hydrazine hydrate (10 ml, 0.20 mol), refluxed for 12 hours, and vacuum rotary evaporated, and the product was recrystallized with methanol and water to obtain the product.
[0062] The third step: the product of the previous st...
Embodiment 2
[0080] metal complex [Ag 2 Br 2 L] n preparation of
[0081] AgBr (0.05mmol, 0.009g), HL-NO 3 (0.05mmol, 0.015g), acetonitrile (1.0ml), ammonia water (0.5ml), and DMAC (1.0ml) were added to the reaction kettle, ultrasonicated for 15min, sealed and heated at 80-100°C for 3-5 days, and then Cool to 30°C at a rate of 3°C per hour. Open the kettle, filter, wash away impurities with a small amount of methanol, and obtain yellow columnar crystals after drying at room temperature. The same operation steps, different solvent combinations: acetonitrile (2ml), ammonia (0.5ml), ethanol (0.5ml); acetonitrile (2.0ml), ammonia (0.5ml), dimethylformamide (0.5ml); acetonitrile (2ml), ammonia (0.5ml), methanol (0.5ml); acetonitrile (2ml), ammonia (0.5ml) dimethyl sulfoxide (0.5ml); acetonitrile (2ml), ammonia (0.5ml) can be combined [Ag 2 Br 2 L] n . Variable temperature powder diffraction pattern as Figure 19 shown, from Figure 19 It can be seen that the complex [Ag 2 Br 2L] ...
Embodiment 3
[0084] metal complex [Ag 2 LCl 2 ] n preparation of
[0085] In the polytetrafluoroethylene liner of a reaction kettle with a volume of 12mL, metal salt AgCl (0.1mmol, 15mg) was added, and the ligand HL-NO 3 (0.1 mmol, 29.8 mg), acetonitrile (4.0 mL) and methanol (2.0 mL), ammonia (1.0 mL), water (1.0 mL). After half an hour of sonication with an ultrasonic instrument, cover the lid of the inner liner and seal the inner liner into the stainless steel shell of the reaction kettle, heat to 120°C and keep the temperature constant for 72 hours, then cool to 30°C at a rate of 3°C / h, filter and After washing with water, yellow columnar crystals were obtained.
[0086] The prepared metal complex [Ag 2 LCl 2 ] n The crystallographic data are shown in Table 2, [Ag 2 LCl 2 ] n Belongs to the monoclinic system P2 1 The / n space group is a simple one-dimensional chain complex, such as Figure 25 shown. Its asymmetric unit contains a proton-removing ligand, two Ag atoms and tw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com