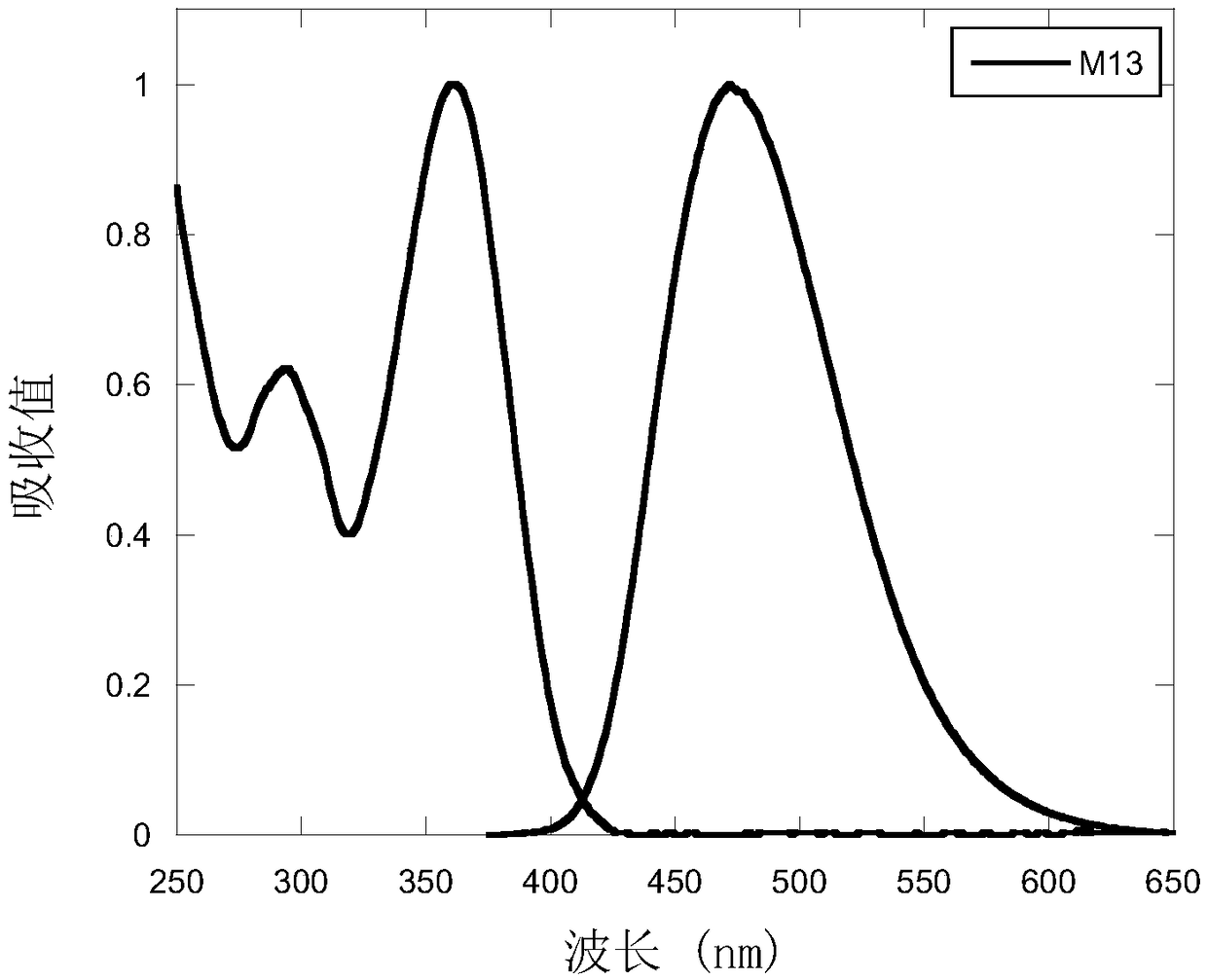
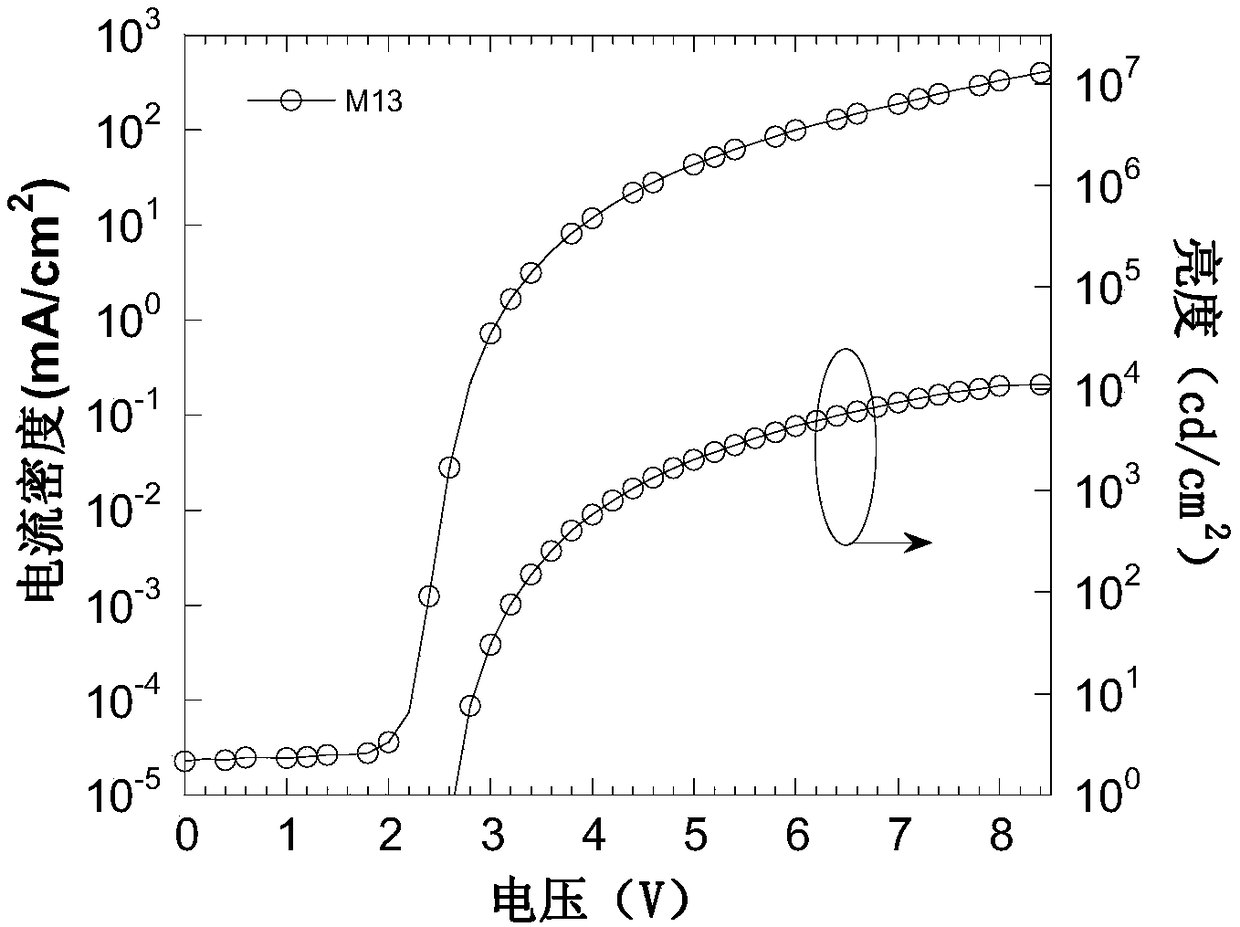
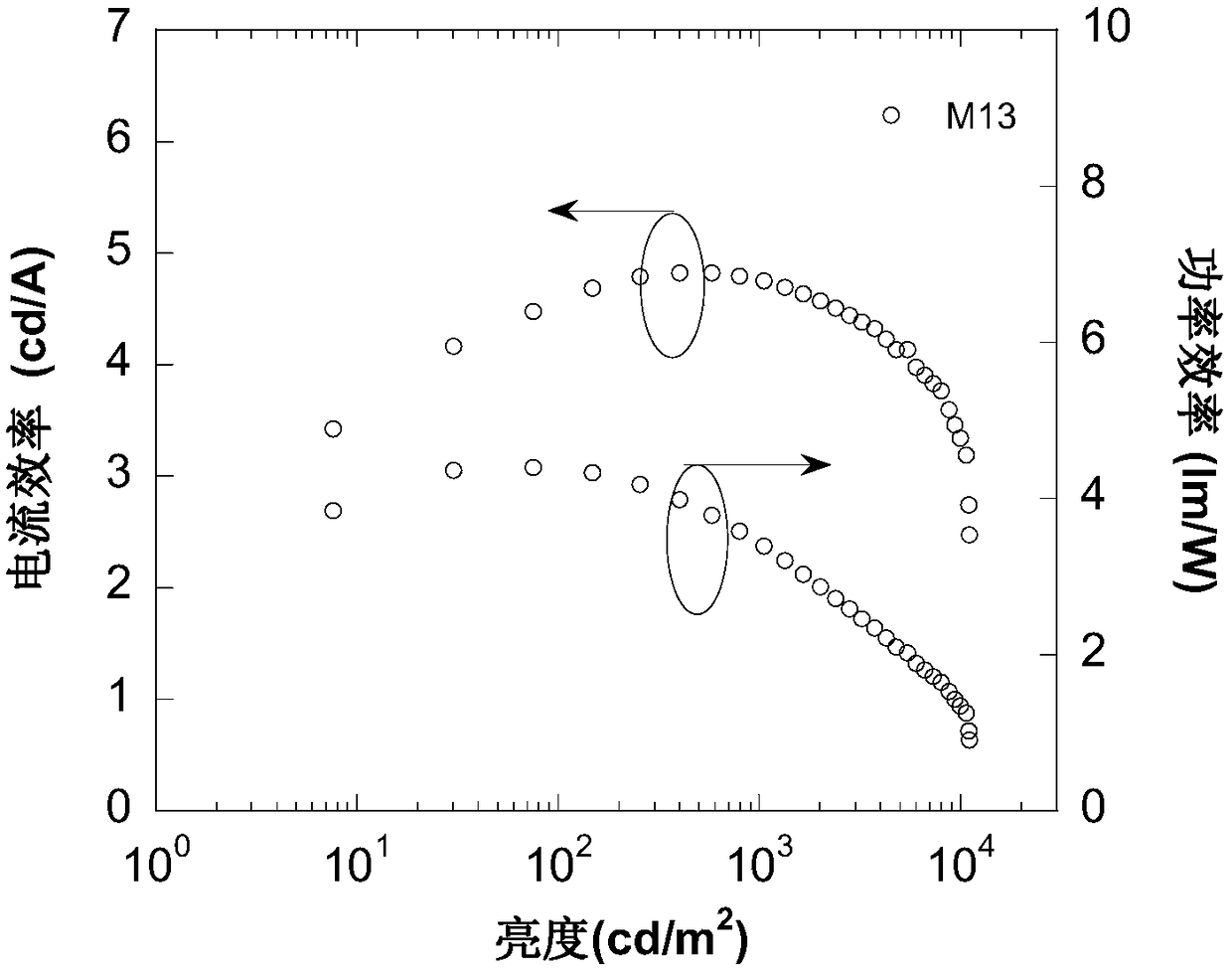
Compound containing 9,9-spiral thioxanthene as well as preparation and application
A compound, thioxanthene technology, applied in the field of electroluminescent materials, achieves the effects of good reproducibility, improved device performance, and determined molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Dissolve 2-bromophenylphenylsulfide (11mmol, 2.93g) in 60ml of anhydrous THF, cool to -78°C, add n-BuLi (14.36mmol) dropwise, keep warm for 1 hour, and 9, 10-Anthraquinone (10mmol, 2.08g) was dissolved in 100ml THF and added dropwise to the reaction system overnight. It was extracted with dichloromethane, dried, and passed through a silica gel chromatography column Br to obtain a white solid. The obtained product was directly added to glacial acetic acid, heated to reflux, and 15 ml of concentrated hydrochloric acid was added to form a solid precipitate, which was filtered by suction to obtain the white solid product M1 (2.7 g, yield 50%). 1 H NMR (500MHz, CDCl 3 ( ddd, J = 8.4, 7.2, 1.4 Hz, 1H), 6.52 (dd, J = 8.1, 1.1 Hz, 1H).
[0030] (2) Dissolve M1 (2.7g, 7.7mmol) in 50mL of glacial acetic acid and heat to reflux. Add 2 times molar equivalent of liquid bromine and react overnight. It was extracted with dichloromethane, washed with water, dried, and the solv...
Embodiment 2
[0035](1) Dissolve 2-bromophenylphenyl ether (11mmol, 2.82g) in 60ml of anhydrous THF, cool to -78°C, add n-BuLi (14.36mmol) dropwise, keep warm for 1 hour, and 2,7 -Dibromothioxanthone (10mmol, 3.70g) was dissolved in 100ml THF and added dropwise to the reaction system overnight. It was extracted with dichloromethane, dried, and passed through a silica gel chromatography column Br to obtain a white solid. The obtained product was directly added to glacial acetic acid, heated to reflux, and 15 ml of concentrated hydrochloric acid was added to form a solid precipitate, which was filtered by suction to obtain the white solid product M4 (2.7 g, yield 50%).
[0036] (2) Add M4 and 50mL glacial acetic acid in a 100mL three-necked flask, after stirring, add 10mL of 30% hydrogen peroxide, heat to reflux overnight, add ethanol after cooling, filter with suction, and remove the solvent under reduced pressure to obtain a white solid M5 with a yield of 97 %. 1 H NMR (500MHz, CDCl 3 )δ...
Embodiment 3
[0040] (1) Dissolve 2-bromotriphenylamine (11mmol, 3.42g) in 60ml of anhydrous THF, cool to -78°C, add n-BuLi (14.36mmol) dropwise, keep warm for 1 hour, and 2,7-di Bromothioxanthone (10mmol, 3.70g) was dissolved in 100ml THF and added dropwise to the reaction system overnight. It was extracted with dichloromethane, dried, and passed through a silica gel chromatography column Br to obtain a white solid. The obtained product was directly added to glacial acetic acid, heated to reflux, and 15 ml of concentrated hydrochloric acid was added to generate a solid precipitate, which was filtered by suction to obtain a white solid product M6 (2.7 g, yield 50%).
[0041] (2) Add M6 and 50mL glacial acetic acid in a 100mL three-necked flask, after stirring, add 10mL of 30% hydrogen peroxide, heat to reflux overnight, add ethanol after cooling, filter with suction, remove the solvent under reduced pressure to obtain a white solid M7, the yield is 97 %. 1 H NMR (500MHz, CDCl 3 )δ: 8.01 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com