Preparation method and application of hydrotalcite-based NiMnTi catalyst
A hydrotalcite-based, catalyst technology, applied in chemical instruments and methods, heterogeneous catalyst chemical elements, physical/chemical process catalysts, etc., can solve the problem of high working temperature, achieve good thermal stability, high N2 selectivity, The effect of large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: c(Ni 2+ ) : c(Mn 2+ ) : c(Ti 4+ ) =3:1:1
[0034] (1) Synthesis of NiMnTi-LDH precursor: Weigh 1.134 g Ni(NO 3 ) 2` 6H 2 O, 1.105 g urea, 0.458 ml butyl titanate, 0.303 ml Mn(NO 3 ) 2 (mass fraction is 50%) dissolved in 100 ml of distilled water, transfer the mixture to a 500 ml round bottom flask, add 3±0.5 ml absolute ethanol dropwise in the round bottom flask with a rubber dropper, and put the round bottom flask Put it into an oil bath magnetic stirring pot, dissolve at 90 °C for 4 h, adjust the temperature of the oil bath to 100 °C, nucleate and crystallize for 48 h, and obtain a turbid solution with a pH value of 7.6. After cooling down to room temperature, the obtained solution was filtered and washed until neutral, and dried overnight in an oven at 60±5° C. to obtain a NiMnTi-LDH precursor.
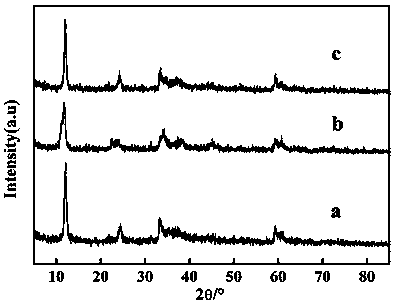
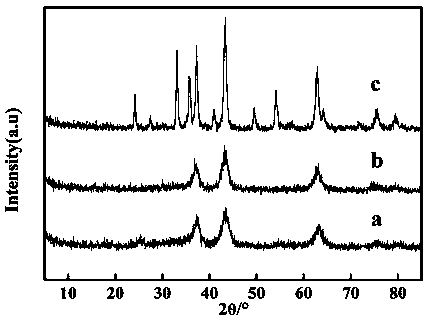
[0035] (2) Carry out X-ray diffraction analysis on the prepared crystal fine powder product, and its diffraction spectrum is as attached figure 1 shown. ...
Embodiment 2
[0040] Example 2: c(Ni 2+ ) : c(Mn 2+ ) : c(Ti 4+ ) =2:2:1
[0041] (1) Synthesis of NiMnTi-LDH precursor: weigh 1.890 g Ni(NO 3 ) 2` 6H 2 O, 1.500 g urea, measure 1.1±0.2 ml butyl titanate with a graduated cylinder, 1.145 ml Mn(NO 3 ) 2 (Mass fraction is 50%) dissolved in 250 ml of distilled water, transfer the mixture to a 500 ml round bottom flask, add 8 ± 0.5 ml absolute ethanol dropwise in the round bottom flask with a rubber dropper, and put the round bottom flask Put it into an oil bath magnetic stirring pot, dissolve at 90 °C for 12 h, adjust the temperature of the oil bath to 100 °C, and nucleate and crystallize for 24 h to obtain a turbid solution with a pH value of 8.2. After cooling down to room temperature, the resulting solution was filtered and washed with water until neutral, and dried overnight in an oven at 60±5°C to obtain a NiMnTi-LDH precursor.
[0042] (2) Carry out X-ray diffraction analysis on the prepared crystal fine powder product, and its di...
Embodiment 3
[0048] Example 3: c(Ni 2+ ) : c(Mn 2+ ) : c(Ti 4+ ) =3:2:1
[0049] (1) Synthesis of NiMnTi-LDH precursor: Weigh 2.269 g Ni(NO 3 ) 2` 6H 2 O, 2.763 g urea, pipette 1.145 ml butyl titanate, and measure 1.213 ml Mn(NO 3 ) 2 (Mass fraction is 50%) dissolved in 340ml distilled water, transfer the mixed solution to a 500 ml round bottom flask, add 10±0.5ml absolute ethanol dropwise in the round bottom flask with a rubber dropper, put the round bottom flask Put it into an oil bath magnetic stirring pot, dissolve at 90 °C for 8 h, adjust the temperature of the oil bath to 100 °C, and nucleate and crystallize for 12 h to obtain a turbid solution, the pH of which is 8.0. After cooling down to room temperature, the resulting solution was filtered and washed with water until neutral, and dried overnight in an oven at 60±5°C to obtain a NiMnTi-LDH precursor.
[0050] (2) Carry out X-ray diffraction analysis on the prepared crystal fine powder product, and its diffraction spectrum ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com