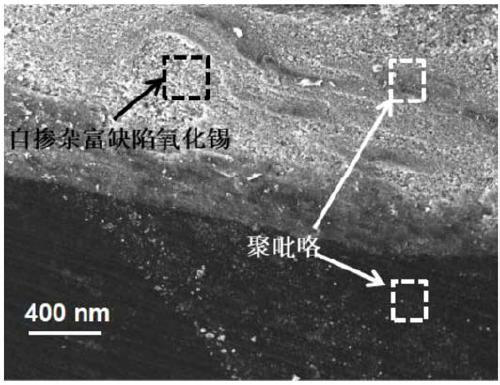
Preparation method of polypyrrole/self-doped defect-rich tin oxide heterojunction nano composite photocatalytic material
A photocatalytic material and nanocomposite technology, which is applied in the field of preparation of nanocomposite photocatalytic materials, can solve the problems of obvious material agglomeration effect, insufficient environmental and friendly reaction raw materials, etc., and achieve simple process control, high efficiency and stable photocatalytic performance, and plasticity. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1) Take 1 mmol of analytically pure stannous pyrophosphate (Sn 2 P 2 O 7 ) and 1.5 mmol of acetic acid (CH 3 COOH) was fully dissolved in 5 mL of absolute ethanol, then 0.5 mmol of alkyldimethylhydroxypropyl phosphobetaine, 2 mmol of tea polyphenols and 13 mL of deionized water were added in turn to obtain solution A. Constant-temperature magnetic stirring was continuously used in an ice-salt bath of calcium chloride and crushed ice at -40°C;
[0028] 2) The solution A was transferred to a polytetrafluoroethylene-lined hydrothermal kettle at a filling ratio of 70%, and then the reaction kettle was placed in a thermostatic oven at 80° C. for 72 hours, and the hydrothermal reaction was completed. Self-doping defect-rich tin oxide heterojunction mixed solution B;
[0029] 3) Control pyrrole (C 4 H 5 N) and stannous pyrophosphate (Sn) used in step 1) 2 P 2 O 7 ) at a molar ratio of 0.01:1, pyrrole (C 4 H 5 N) fully dissolve in absolute ethanol in a closed contain...
Embodiment 2
[0032] 1) Take 1 mmol of analytically pure stannous pyrophosphate (Sn 2 P 2 O 7 ) and 2.5 mmol of acetic acid (CH 3 COOH) is fully dissolved in 10mL of absolute ethanol, then add 5mmol of alkyl dimethyl hydroxypropyl phospholipid betaine, 8mmol of tea polyphenols and 18mL of deionized water and mix to obtain solution A, the whole process is in - Constant-temperature magnetic stirring was continuously used in an ice-salt bath of calcium chloride and crushed ice at 30°C;
[0033] 2) The solution A was transferred to a polytetrafluoroethylene-lined hydrothermal kettle at a filling ratio of 66%, then the reaction kettle was placed in a constant temperature oven and kept at 130 ° C for 60 h, the hydrothermal reaction was completed, and cooled to room temperature to obtain a solution containing Self-doping defect-rich tin oxide heterojunction mixed solution B;
[0034] 3) Control pyrrole (C 4 H 5 N) and stannous pyrophosphate (Sn) used in step 1) 2 P 2 O 7 ) at a molar rati...
Embodiment 3
[0038] 1) Take 1 mmol of analytically pure stannous pyrophosphate (Sn 2 P 2 O 7 ) and 3.6 mmol of acetic acid (CH 3 COOH) was fully dissolved in 16mL of absolute ethanol, followed by adding 8mmol of alkyl dimethyl hydroxypropyl phospholipid betaine, 15mmol of tea polyphenols and 23mL of deionized water and mixed uniformly to obtain solution A, the whole process was in - Continuously use constant temperature magnetic stirring in an ice-salt bath of calcium chloride and crushed ice at 20°C;
[0039] 2) Transfer solution A to a polytetrafluoroethylene-lined hydrothermal kettle at a filling ratio of 35%, then put the reaction kettle into a constant temperature oven and keep it at 160°C for 48 hours. After the hydrothermal reaction is completed, it is cooled to room temperature to obtain a Self-doping defect-rich tin oxide heterojunction mixed solution B;
[0040] 3) Control pyrrole (C 4 H 5 N) and stannous pyrophosphate (Sn) used in step 1) 2 P 2 O 7 ) at a molar ratio of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com