A kind of α-amylase gene and its application
A technology of amylase and gene, which is applied in genetically engineered bacteria and its application fields, can solve the problems of poor enzyme activity, instability, and insufficient purity of α-amylase, and achieve the effects of short cycle, easy purification, and large-scale expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Nucleotide sequence of amylase gene isolated from Bacillus subtilis 48-1.
[0029] Genomic DNA of Bacillus subtilis was extracted by the CTAB method, and 2 μl was used as a template for polymerase chain reaction (PCR). According to the conserved region of the published amylase homologous sequence, primers (primer F and primer R) were designed in combination with the vector used. , the primers, components and amplification conditions used in this PCR reaction process are as follows:
[0030] F: 5'-GGAATTCCATATGGTGAGAAGCAAAAAAA-3' (SEQ ID NO.3)
[0031]R: 5'-CGGGATCCTTATTGTGCAGCTGCTTGTA-3' (SEQ ID NO.4)
[0032] The composition of the PCR amplification system is shown in Table 1.
[0033] Table 1 The reaction parameters of PCR
[0034] 10×Taq Buffer 5μl dNTP (2.5mmol / L) 4μl Template (genomic DNA) 2μl Primer F 2μl Primer R 2μl Taq DNA polymerase 0.5μl wxya 2 o
[0035] Amplification conditions: pre-denatur...
Embodiment 2
[0036] Embodiment 2: Construction of recombinant expression vector
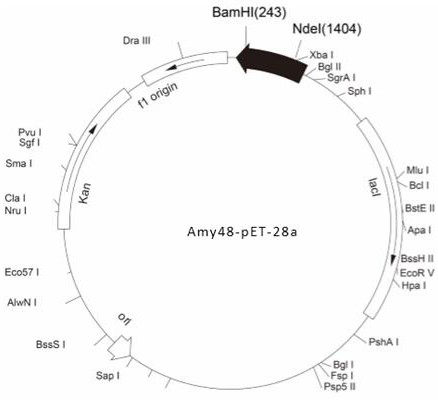
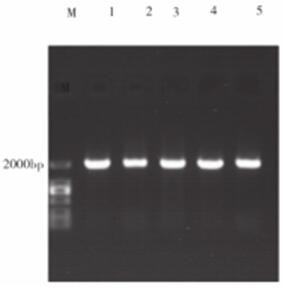
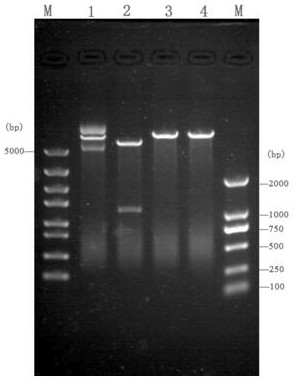
[0037] The fragment amplified in Example 1, because the 5' end of the primer F contains the Nde I restriction site (the 8th base to the 13th base) and the 5' end of the primer R contains the BamH I respectively Restriction site (from the 3rd base to the 8th base), so use these two enzymes to double-enzyme digest the fragment, and pET28a is double-enzyme-digested with the same enzyme at the same time, and the large fragment is recovered by electrophoresis. And use T4 ligase to ligate at 16°C for 6h, the schematic diagram of the successfully constructed vector is as follows figure 1 shown. The ligation product was transformed into Escherichia coli BL21(DE3) by chemical transformation method, cultured on LB solid plate containing kanamycin (100 μg / ml), and the white colonies growing on the plate were picked and analyzed by colony PCR ( figure 2 ) and extracted plasmid single and double digestion ( image 3 )...
Embodiment 3
[0038] Embodiment 3: the preparation of amylase
[0039] The recombinant engineering bacterium obtained in Example 2 is connected to 100ml LB liquid medium (containing 1‰50mg / ml kanamycin) by 1% inoculum, 37 DEG C, 150rpm is cultivated to OD600=0.6, adds 1‰ of 1mM IPTG (isopropyl-β-D-thiogalactopyranoside), transferred to 16 ° C, 80rpm after induction for 10h, centrifuged to collect the bacteria, the bacteria were washed with 5ml 50mmol / l imidazole buffer (pH8.2 ) suspension, ultrasonic crushing. Centrifuge to take supernatant and precipitate respectively, then suspend the precipitate with 5ml 50mmol / l imidazole buffer (pH8.2), take 50μl respectively for SDS polyacrylamide gel electrophoresis (SDS-PAGE), bands appear in the precipitate, indicating The resulting recombinant protein is an inclusion body, such as Figure 4 Lane 3 shows. Example 4: Denaturation and renaturation of inclusion bodies and determination of enzyme activity
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com