Site-directed mutagenesis-modified agarase mutant with improved thermal stability
A thermostability and site-directed mutation technology, which is applied in the fields of genetic engineering and enzyme engineering, can solve the problems of agarase instability and limit the application of agarase, and achieve the effect of increasing half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Construction of agarase mutant D622G
[0026] The gene sequence of the mutant was amplified by overlap extension PCR technique. First design degenerate primers to amplify the source Vibiro The conserved sequence of the agarase rAgaZC-1 gene of sp. was cloned by TAIL-PCR to obtain the agarase gene aga ZC-1 full-length sequence (sequence shown in SEQ ID NO.1); construction of recombinant plasmid pET-22b- aga ZC-1. The recombinant plasmid pET-22b- aga ZC-1 was used as the template for the first round of PCR amplification to aga ZC-1-F, D622G-R as primers to amplify the upstream fragment of the target gene to aga ZC-1-R, D622G-F are primers to amplify the downstream fragment of the target gene; then the purified upstream and downstream fragments are mixed and properly diluted as the template for the second round of PCR to aga ZC-1-F, aga ZC-1-R is the primer for amplifying the target gene. The information of each primer is as follows, where the und...
Embodiment 2
[0036] Example 2 Induced expression and purification of agarase
[0037]The engineered bacteria expressing rAgaZC-1 and mutant enzyme D622G were inoculated into LB medium containing 100 mg / L Amp, cultured overnight at 37°C and 200 rpm, and then transferred to the same medium with 1% inoculum. Continue culturing until OD600 is about 0.6, then add IPTG at a final concentration of 1 mM, and continue culturing at 28°C and 180 rpm for 8 h.
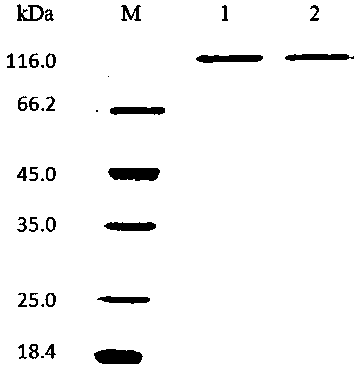
[0038] The fermentation broth was centrifuged at 12,000 rpm for 10 min at 4°C, and the supernatant was concentrated by using a hollow fiber column (MWCO: 10 kDa) and ammonium sulfate precipitation to concentrate the enzyme solution. Ammonium sulfate was removed by dialysis in the buffer solution, and the target protein was purified by NTA-Ni column (BBI, China) after filtration through a 0.22 μm membrane. After the collected enzyme solution was concentrated properly, SDS-PAGE was carried out, and the results were as follows: Figure 2-A As sh...
Embodiment 3
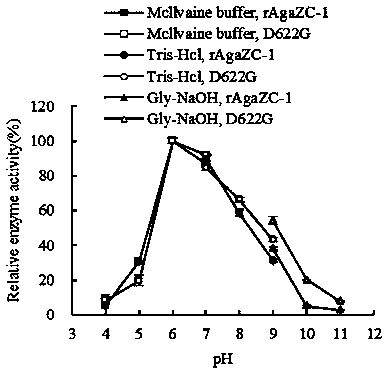
[0039] Example 3 Characterization of agarase
[0040] 1. Agarase Activity Assay
[0041] The activity of agarase was determined by DNS method. Add 100 μL of enzyme solution to 900 μL of 50 mM Tris-HCl (pH 7) solution containing 0.2% agar, and react in a water bath at 40°C for 15 min, then add 1.5 mL of DNS reagent to terminate the reaction. After developing color in boiling water for 5 min, cool to room temperature, shake well and measure OD 540 . The released reducing sugar content was determined according to the glucose standard curve. Definition of enzyme activity unit: Under certain reaction conditions, the amount of enzyme required to hydrolyze agarose to produce 1 μmol reducing sugar per minute is defined as an enzyme activity unit U.
[0042] 2. Catalytic Kinetics of Agarase
[0043] At 38.5°C, the enzyme activity of the pure enzyme at different substrate concentrations (0.1-0.5 g / L) was determined, and the hydrolysis reaction of the pure enzyme catalyzed by the ag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com