3,4-dihydroxyacetophenone derivative, preparation method, application and pharmaceutical composition thereof
A derivative, the technology of qingxinone, which is applied in the direction of drug combination, carbon-based compound preparation, organic compound preparation, etc., can solve the problems restricting clinical application and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
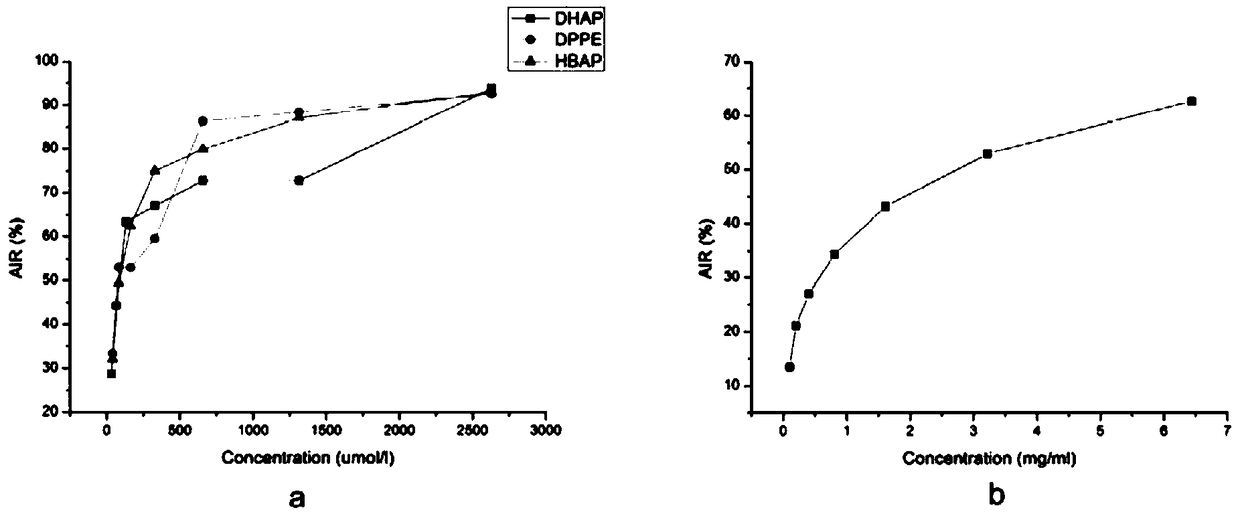
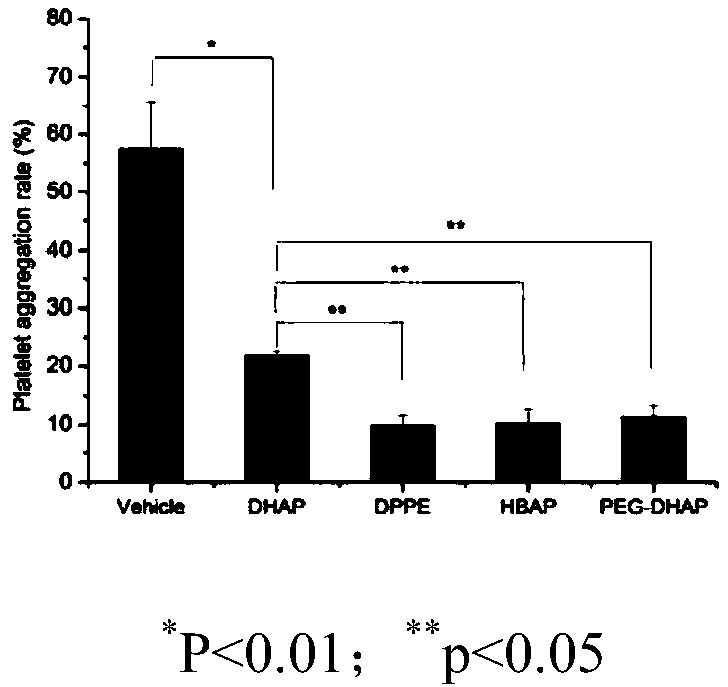
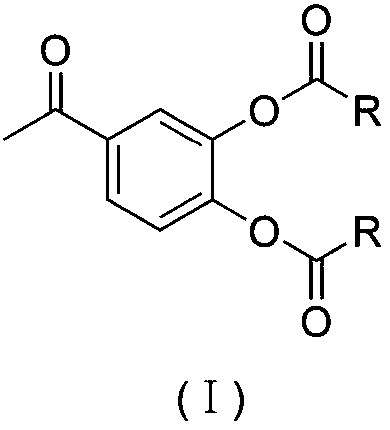
[0063] Example 1 Preparation of 3,4-dihydroxyacetophenone fatty acid ester derivative (I)
[0064] The preparation method of the derivative comprises the following steps: the feeding concentration of 3,4-dihydroxyacetophenone is 0.01-0.1mol / L; the molar ratio of 3,4-dihydroxyacetophenone, acid chloride and triethylamine is 1 : 2~8: 2~8, the reaction temperature is 10~100°C, the reaction time is 3~10 hours, the reaction process is monitored by high performance liquid chromatography, ODS C18 chromatographic column (250×4.60mm, 5μm), sample loading Volume 20μL, flow rate 1mL / min, column temperature at room temperature, mobile phase composition, methanol / water (60:40, volume ratio), 254nm ultraviolet detection; after the reaction, filter to obtain the filtrate; the filtrate was evaporated to dryness under reduced pressure, and ethyl acetate was added Dissolved, washed with water and saturated NaCl solution successively. The ethyl acetate phase was dried overnight with anhydrous m...
Embodiment 2
[0073] Example 2 Preparation of 3,4-dihydroxyacetophenone benzyl ether derivative (II)
[0074] The preparation method of the derivative comprises the following steps: the feeding concentration of 3,4-dihydroxyacetophenone is 0.1~1mol / L, 3,4-dihydroxyacetophenone, 4-substituted benzyl bromide and anhydrous K 2 CO 3 The molar ratio is 1:0.5~2:0.5~2, the reaction temperature is 10~100°C, the reaction time is 1~5 hours, and the reaction process is monitored by high performance liquid chromatography, ODS C18 chromatographic column (250×4.60mm, 5μm), sample volume 20μL, flow rate 1mL / min, column temperature at room temperature, mobile phase composition, methanol / water (60:40, volume ratio), 254nm UV detection. After the reaction was completed, the filtrate was obtained by filtration. The filtrate was diluted with five times the volume of ethyl acetate, washed with water and saturated NaCl solution successively. The ethyl acetate phase was dried overnight with anhydrous magnesium...
Embodiment 3
[0083] Example 3 Preparation of the polyethylene glycol sustained-release body (Ⅲ) of 3,4-dihydroxyacetophenone
[0084] The preparation method of this derivative comprises the steps:
[0085] In the first step, 3,4-dihydroxyacetophenone (also known as Qingxinone) is used as a starting material in anhydrous organic solvents including acetonitrile, N,N-dimethylformamide, acetone, dichloro Among methane and methyl tert-butyl ether, the following basic reagents include triethylamine, pyridine, 4-dimethylaminopyridine, anhydrous K 2 CO 3 As a catalyst, react with dianhydride to obtain the corresponding intermediate products (IV) and (V).
[0086] In the second step, N-hydroxysuccinimide and diphenylphosphinyl chloride, in the following anhydrous organic solvents including acetonitrile, N,N-dimethylformamide, acetone, dichloromethane, methyl tert-butyl In ether, the following basic reagents include triethylamine, pyridine, 4-dimethylaminopyridine, anhydrous K 2 CO 3 As a catal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com