Recombinant equine chorionic gonadotropin fusion protein as well as preparation method and application thereof
A chorionic gonadotropin and recombinant protein technology, applied in the field of biomedicine and animal reproduction, can solve problems such as mare abortion, PMSG animal husbandry production restrictions, and brutal methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1. Obtaining of recombinant reCG and reCG-Fc fusion protein
[0109] In order to obtain active recombinant eCG protein products, reCG and reCG-Fc fusion proteins were simultaneously designed: search eCGα (GenBank NM_001099763.1), β (GenBank NM_001197093.1) and human Fc (GenBank AH005273.2) in GenBank The gene sequence of the α subunit, β subunit and β-Fc subunit gene sequence was optimized according to the mammalian codon bias, and the optimized α subunit nucleotide sequence is shown in SEQ ID NO: 2 in the sequence table, The optimized β subunit nucleotide sequence is shown in SEQ ID NO:4 in the sequence listing, and the optimized β-Fc subunit nucleotide sequence is shown in SEQ ID NO:6 in the sequence listing.
[0110] The nucleotide sequences shown in SEQ ID NO:2, SEQ ID NO:4 and SEQ ID NO:6 in the artificially synthesized sequence listing.
[0111] The artificially synthesized above-mentioned nucleotide sequences, that is, the nucleotide coding sequences of ...
Embodiment 2
[0118] Example 2 Fermentation and purification of recombinant reCG-Fc fusion protein
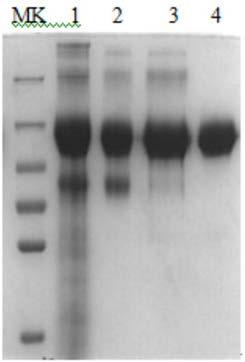
[0119] The reCG-Fc stably transfected cell line prepared in Example 1 was cultured in a shake flask, and the cell culture conditions were optimized. The optimized conditions showed that 100 μM Cu was added to the basal medium 2+ , adding 2mM N-acetyl-D-mannosamine to the feed medium can increase the degree of glycosylation of recombinant reCG-Fc and increase the content of sialic acid by about 20%. After the culture conditions of the shake flask cells were optimized successfully, they were scaled up in a 7L bioreactor and cultured to express the recombinant reCG-Fc fusion protein.
[0120] Cultivate the expressed reCG-Fc fusion protein cell culture fluid, remove cells and cell debris through a two-stage deep filter membrane bag, and then filter with a 0.22 μm filter membrane to obtain a clarified fermentation broth. The clarified fermentation broth was first purified by weak cation exchange...
Embodiment 3
[0121] Example 3 Activity Assay of Recombinant reCG-Fc Fusion Protein
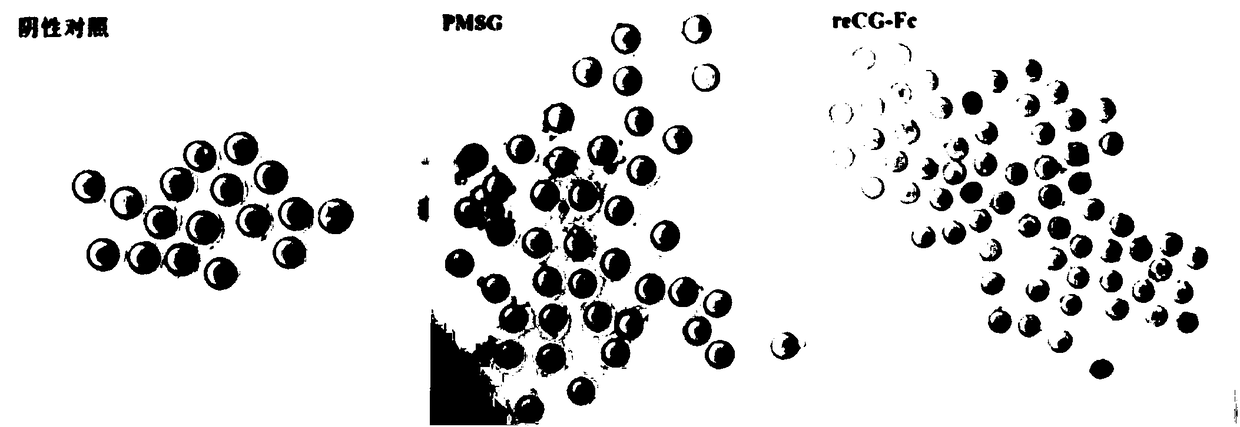
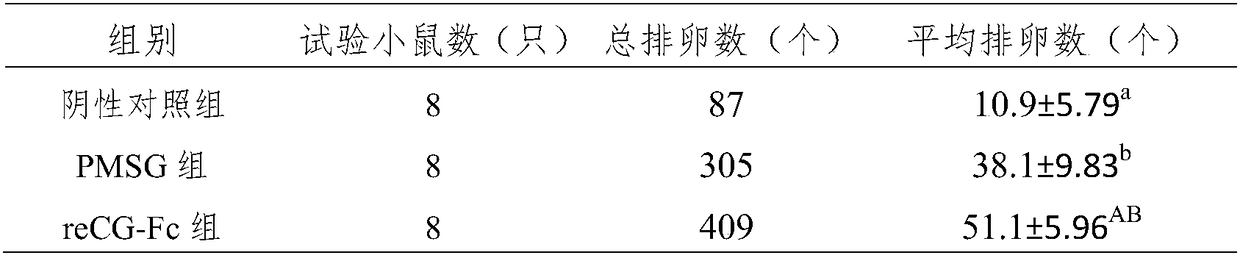
[0122] The biological activity of recombinant reCG-Fc was measured by the rat ovary weight gain method (Steelman-Pohley method), and the drug activity was tested by referring to the "blood gonadotropin bioassay method" in the "Quality Standards for Veterinary Drugs", and the commercially available PMSG was used as the standard. . The specific implementation is as follows: Recombinant reCG-Fc (estimated specific activity 10000U / mg) and PMSG are formulated into three doses of 40IU, 20IU and 10IU, high, medium and low. Female SD (Sprague Dawley) rats aged 21-23 days and weighing 40-55 g were randomly divided into 6 groups, 6 rats in each group. Each rat was subcutaneously injected with 0.5ml of the corresponding drug, and after 6 days, the rat was killed, weighed, dissected, the ovary was removed, weighed, and converted into the weight of the ovary per 100g body weight. The specific activity of reCG-Fc calc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com