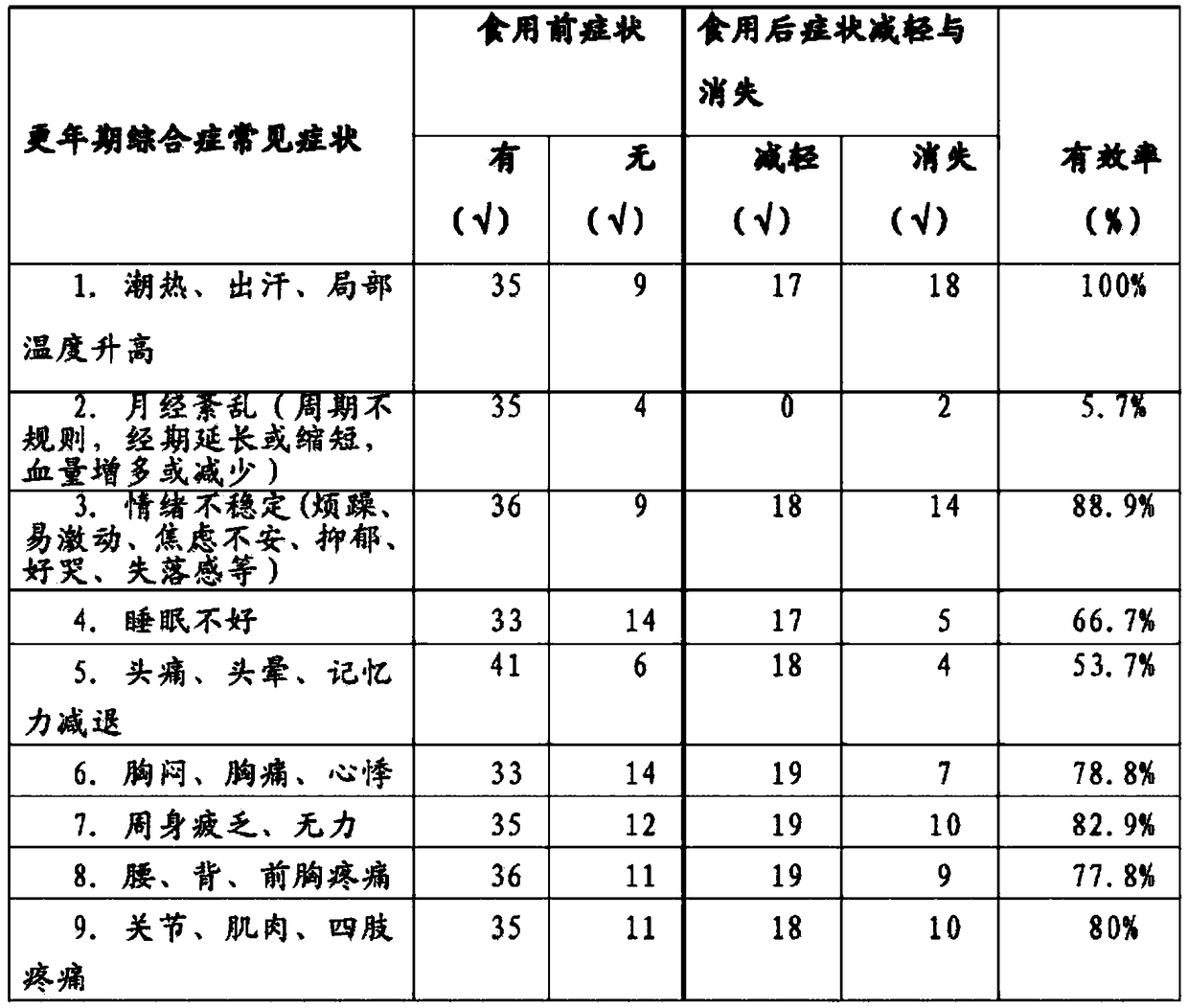
NADH-containing (reduced nicotinamide adenine dinucleotide-containing) composition and preparation to improve climacteric symptoms and their preparation methods and applications
A composition and symptomatic technology, which is applied in the fields of medicine and health care, can solve the problems of large toxic and side effects, hyperplasia of endometrium, uterine bleeding, etc., achieve low production cost, easy large-scale production, and improve the effect of menopausal symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
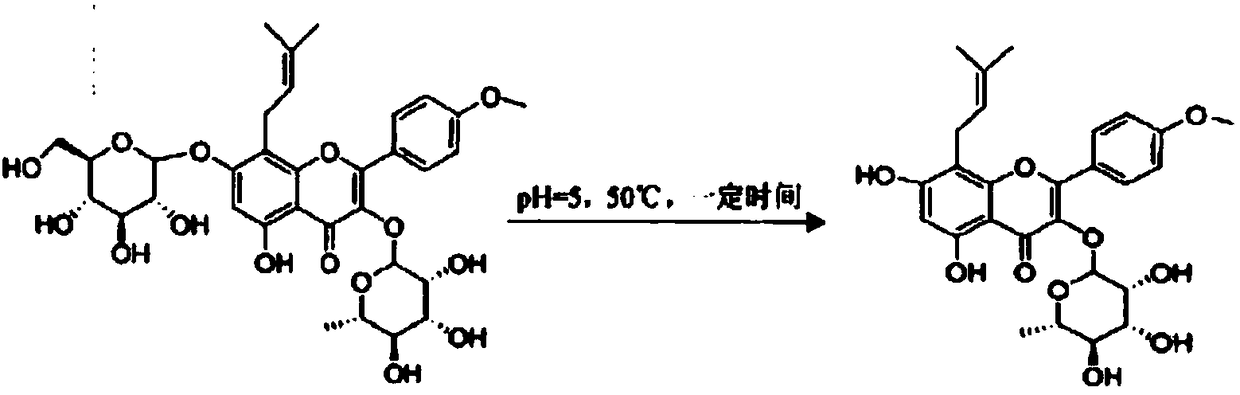
[0028] Among them, NADH is nicotinamide adenine dinucleotide (reduced nicotinamide adenine dinucleotide), also known as reduced coenzyme I, with a chemical formula of C21H27N7O14P2 and a molecular weight of 663.43. Its oxidized form is nicotinamide adenine dinucleotide (oxidized nicotinamide adenine dinucleotide, abbreviated as NAD), which plays the role of transferring hydrogen and electrons in biological oxidation, and plays an important role in maintaining cell growth, differentiation and energy metabolism. It participates in the metabolism, rhythm, pathological changes, aging and apoptosis of body cells and other life processes. The current NADH preparation method includes the following steps: extracting the crude extract of nicotinamide riboside adenylyltransferase or its pure enzyme; immobilizing the crude extract of recombinant nicotinamide ribosyl adenylyltransferase or its pure enzyme; The recombinant nicotinamide riboside adenylyltransferase catalyzes the preparation...
Embodiment 1
[0043] (1) Raw materials: 5 parts by weight of NADH, 5 parts by weight of Epimedium secondary aglycone, 5 parts by weight of chondroitin sulfate, 5 parts by weight of ursodeoxycholic acid, 5 parts by weight of bird's nest acid, 5 parts by weight of parts of s-adenosylmethionine, 5 parts of Zhiyuan, 5 parts of DHA, and 5 parts of γ-aminobutyric acid.
[0044] (2) Accessories:
[0045] Including the following parts by weight: 15 parts of Shichangpu, 8 parts of Radix Paeoniae Rubra, 30 parts of Baiziren, 20 parts of theanine, 20 parts of Anemarrhena, 10 parts of Centella asiatica, 15 parts of ginseng leaves, 5 parts of Jiji, 10 parts of silver thread grass, 5 dragon boat flowers; accessories also include parkin complex ester, fumaric acid, fumed silica.
[0046] (3) Preparation method:
[0047] First, pass the dried raw and auxiliary materials through a 30-80 mesh sieve, so that the particle size distribution is uniform, the fluidity is better, and the raw and auxiliary materia...
Embodiment 2
[0050] (1) Raw materials: 10 parts by weight of NADH, 1 part by weight of Epimedium secondary aglycone, 10 parts by weight of chondroitin sulfate, 1 part by weight of ursodeoxycholic acid, 10 parts by weight of bird's nest acid, 1 part by weight of parts of s-adenosylmethionine, 10 parts by weight of Zhiyuan, 1 part by weight of DHA, and γ-aminobutyric acid of 10 parts by weight
[0051] (2) Accessories:
[0052] Including the following parts by weight: 4 parts of Shichangpu, 20 parts of Radix Paeoniae Rubra, 20 parts of Baiziren, 30 parts of theanine, 10 parts of Zhimu, 20 parts of Centella asiatica, 4 parts of ginseng leaves, 12 parts of Jiji, 5 parts Silver thread grass, 10 parts of dragon boat flower, 5 parts of lingxiaohua, 10 parts of wheat bottle grass, 4 parts of North China columbine, 8 parts of deergrass, 3 parts of Spatholobus yunnanensis, 20 parts of medlar, 7 parts of rehmannia, 15 parts Angelica, 4 parts of Cuscuta, 15 parts of Anemarrhena, 7 parts of Morinda of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com