Orthoester 5-fluorouracil prodrug molecule, preparation method and acid-sensitive nanoparticle and application thereof
A fluorouracil and orthoester technology, which can be used in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. The effect of reducing toxic and side effects, good acid degradation performance, and improving lipophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
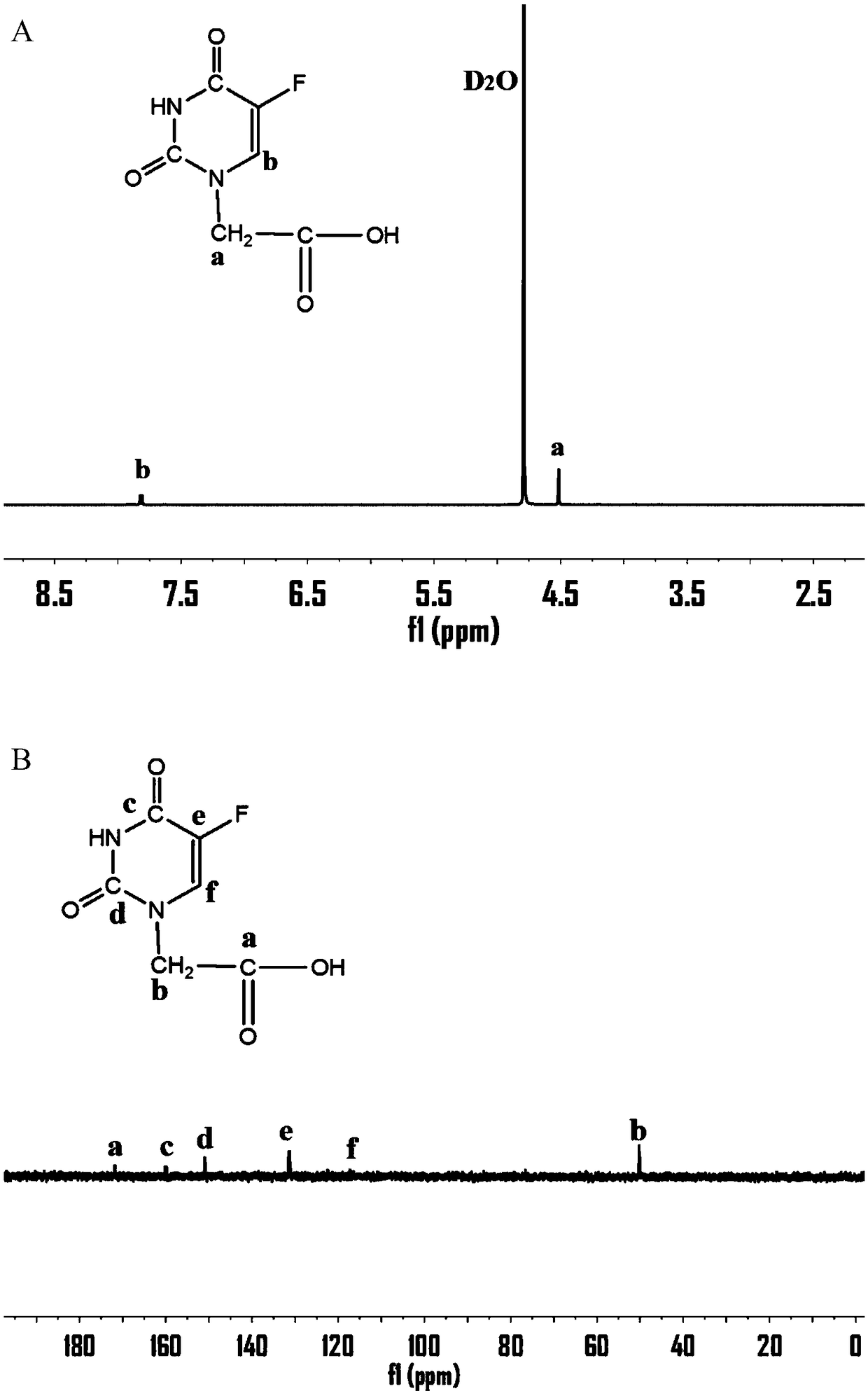
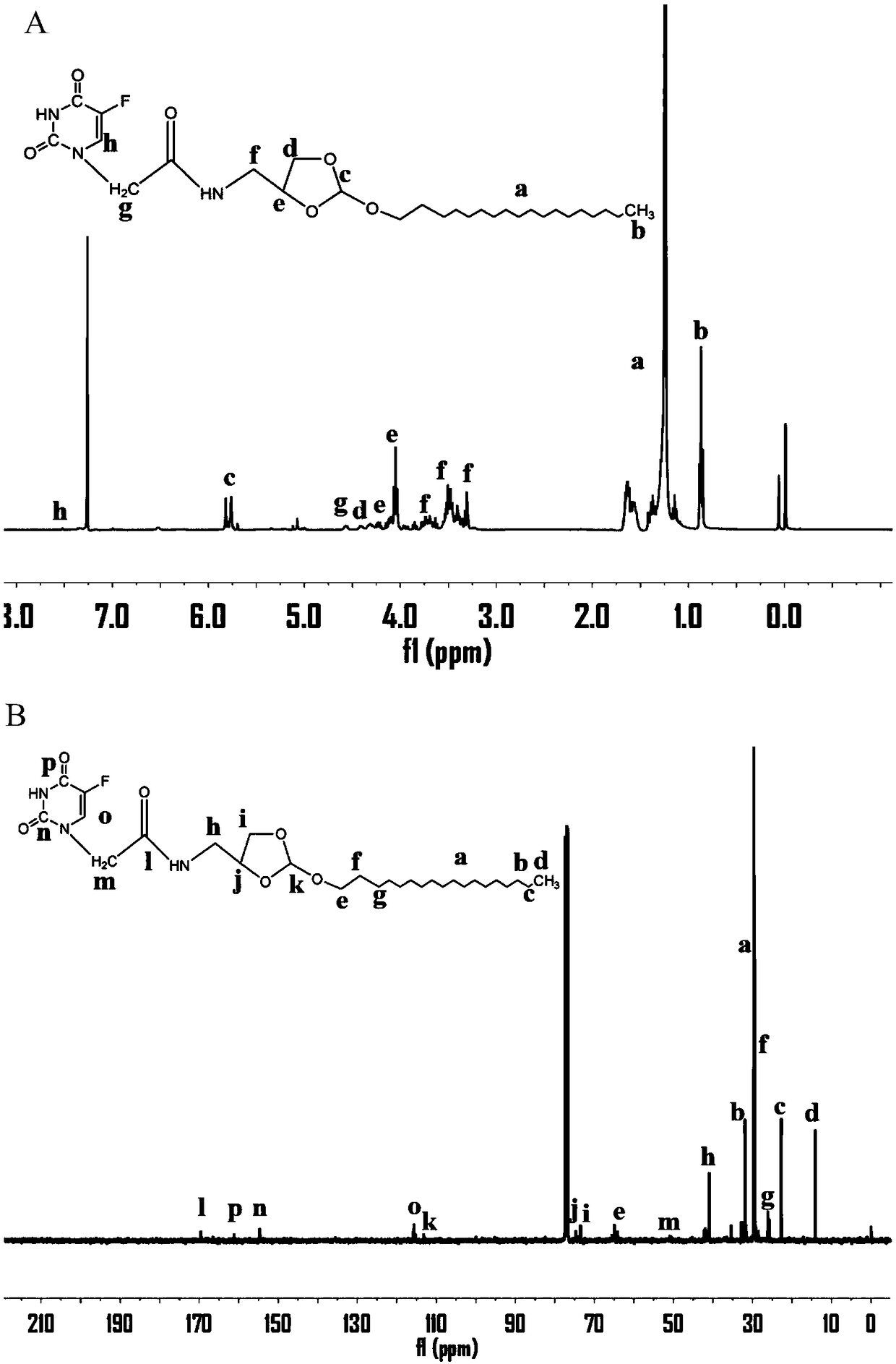
[0039] Preparation of 5-fluorouracil-1-acetic acid:
[0040] Weigh 10.07g of 5-fluorouracil (77.41mmol) into a 250ml round bottom bottle, then add 13.03gKOH (0.23mol), add a small amount of water, react at 100°C for half an hour and then the temperature drops to 60°C, weigh 10.97g of chlorine Acetic acid (0.12mol), dissolved with a small amount of water, placed in the dropping funnel and slowly dripped into the original bottom bottle, reacted for 6 hours, after the reaction, adjusted the pH to 5.0 with hydrochloric acid, cooled at 4°C for 4 hours, and used a sand core funnel Filter to remove the precipitate, adjust the pH to 2.0 with hydrochloric acid, cool in the refrigerator overnight, collect the precipitate, adjust the pH to 5.0 with saturated NaHCO3, cool for 4 hours, filter, adjust the pH of the filtrate to 2.0 with hydrochloric acid, and place in the refrigerator , the precipitate was collected and dried in a vacuum oven to obtain 14.3 g of a white solid product with a ...
Embodiment 2
[0042] Preparation of 2,2,2-trifluoro-N-(2-methoxy-[1,3]dipentane-4-methylene)acetamide:
[0043] (1) Under the protection of nitrogen, take a 250ml round-mouth reaction bottle as a reaction vessel, add 15.49g (0.17mol) 3-amino-1,2-propanediol to it, and dissolve it with 100ml of acetonitrile, ice-bath, and slowly add 36.23 g of ethyl trifluoroacetate, stirred and reacted at room temperature for 12h, after the reaction was completed, the solvent was removed by rotary evaporation, and then 300ml of ethyl acetate was added to dissolve the product after the rotary evaporation, and 100ml of 10% potassium ammonium sulfate solution and 100ml of saturated sodium chloride The solution was washed once respectively, the organic phase was collected, read-dried with anhydrous sulfuric acid, filtered, and the filtrate was concentrated under vacuum to obtain the colorless oily compound 2,2,2-trifluoro-N-(2,3-dihydroxypropane Alcohol) acetamide 26.53g, productive rate is 83.4%;
[0044] (2)...
Embodiment 3
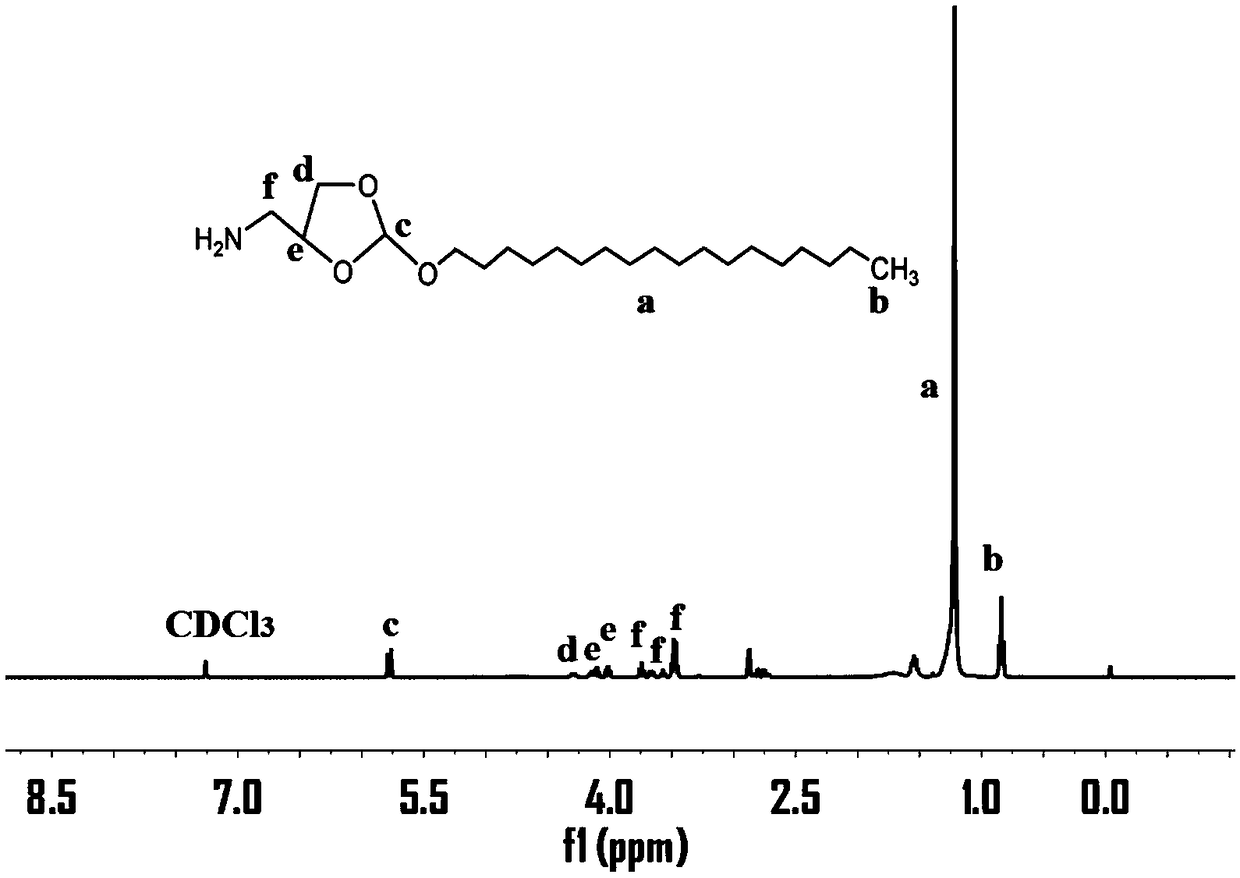
[0046] (1) 2-octalkoxy-(1,3) dioxolane-4-methylamine (C 8- oe-NH 2 ) preparation:
[0047] Take 3.08g of n-octanol (23.65mmol), 8.12g of 2,2,2-trifluoro-N-(2-methoxy-[1,3]dipentane-4-methylene prepared in Example 2 Base) acetamide (35.5mmol) and 118.87mg pyridine p-toluenesulfonate (0.47mmol) were added to a 100ml round bottom reaction flask, reacted in a vacuum oil bath at 130°C for 8h, cooled to room temperature, and terminated by adding a few drops of triethylamine For the reaction, dissolve the reactant with a small amount of dichloromethane, settle in ethanol with a few drops of triethylamine, place it in a -20°C refrigerator for 1 hour, filter it with a sand core funnel, take the upper precipitate, and vacuumize to obtain a white reactant 7.34 g, yield 90.2%, put 7.34g of white reactant in a round-bottomed bottle, dissolve in THF with added triethylamine, dissolve 7.67g of NaOH (0.19mol) in 100ml of deionized water, add in THF after cooling and stir Overnight, remove ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com