Benzisothiazolinone isoxazole acetamide derivative, synthesis method and application thereof
A technology of benzisothiazolinone acetic acid and benzisothiazolinone, which is applied in the field of benzisothiazolinone isoxazole acetamide derivatives and their synthesis, can solve the problems of lack of independent innovation and achieve reaction Mild conditions, simple reaction operation, and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
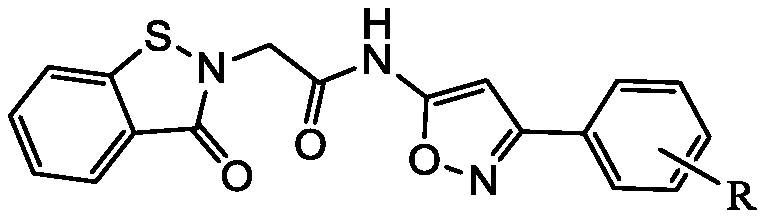
[0026] Example 1 Synthesis of 2-(benzisothiazolin-3-one-2-yl)-N-(3-phenylisoxazol-5-yl)acetamide
[0027] In a 100ml four-necked flask, 3-phenyl-5-aminoisoxazole and benzisothiazolin-3-one-2-ylacetic acid were reacted in a molar ratio of 1:1.1, and DMF(N,N- Dimethylformamide) can be dissolved, add HOBT (1-hydroxybenzotriazole) and EDCI [1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride], HOBT , The molar ratio of EDCI to 3-phenyl-5-aminoisoxazole is 1:1:1, react at 25°C for 26h, and the reaction is completed. The solution was poured into water, and a large amount of solid precipitated out. Filtration, alkali washing, acid washing, water washing. After drying, the crude product was obtained, and the target compound was obtained by column chromatography (ethyl acetate:petroleum ether=1:1.1). Yield: 68.5%. Melting point: 189~191℃. The structural formula and spectrum analysis of the product are as follows:
[0028]
[0029] 1 H NMR (DMSO-d6, 300MHz): 5.27(s,2H...
Embodiment 2
[0036] Synthesis of 2-(Benzisothiazolin-3-on-2-yl)-N-(3-(2-chlorophenyl)isoxazol-5-yl)acetamide
[0037] In a 100ml four-necked flask, 3-o-phenylchloro-5-aminoisoxazole and benzisothiazolin-3-ketone-2-ylacetic acid were reacted in a molar ratio of 1:1.2, with DMF (N, N-dimethylformamide) can be dissolved, add HOBT (1-hydroxybenzotriazole) and EDCI [1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride] , The molar ratio of HOBT, EDCI and 3-o-phenylchloro-5-aminoisoxazole is 1.3:1.2:1, react at 20°C for 30h, and the reaction is completed. The solution was poured into water, and a large amount of solid precipitated out. Filtration, alkali washing, pickling, water washing. After drying, a crude product was obtained, and the target compound was obtained by column chromatography (ethyl acetate:petroleum ether=1:1.3). Yield: 65.2%. Melting point: 174-175°C. The structural formula and spectrum analysis of the product are as follows:
[0038]
[0039] 1H NMR (DMSO-d6,...
Embodiment 3
[0046] Synthesis of 2-(Benzisothiazolin-3-on-2-yl)-N-(3-(4-fluorophenyl)isoxazol-5-yl)acetamide
[0047] In a 100ml four-necked flask, 3-p-fluorophenyl-5-aminoisoxazole and benzisothiazolin-3-ketone-2-ylacetic acid were reacted in a molar ratio of 1:1.2, with DMF (N, N-dimethylformamide) can be dissolved, add HOBT (1-hydroxybenzotriazole) and EDCI [1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride] , the molar ratio of HOBT, EDCI and 3-p-fluorophenyl-5-aminoisoxazole is 1.4:1.1:1, react at 25°C for 32h, and the reaction is completed. The solution was poured into water, and a large amount of solid precipitated out. Filtration, alkali washing, acid washing, water washing. After drying, a crude product was obtained, and the target compound was obtained by column chromatography (ethyl acetate:petroleum ether=1:1.3). Yield: 65.2%. Melting point: 174-175°C. The structural formula and spectrum analysis of the product are as follows:
[0048]
[0049] 1 H NMR (DMS...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com