Preparation method of nickel cobalt lithium aluminate cathode material and lithium ion battery
A technology of nickel cobalt lithium aluminate and positive electrode material, which is applied to batteries, battery electrodes, secondary batteries, etc., can solve the problems of decreased rate performance of positive electrode materials, poor ionic conductivity of the coating layer, and poor lithium ion conductivity, etc. Achieve the effect of inhibiting decomposition and gas production, inhibiting side reactions, and reducing the amount of residual lithium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
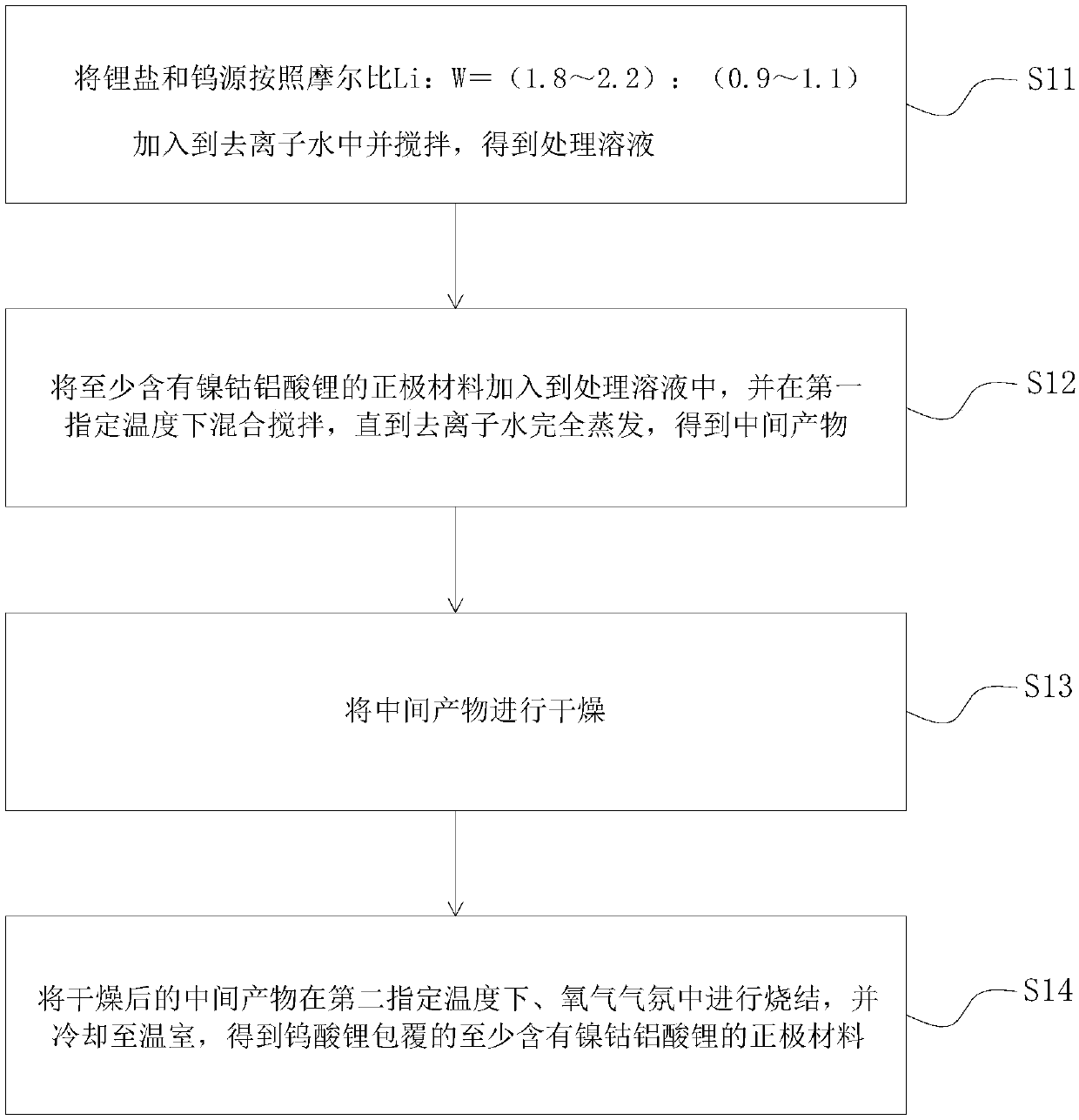
[0042] refer to figure 1 , the embodiment of the present invention proposes a method for preparing a nickel-cobalt-lithium-aluminate positive electrode material, comprising the following steps:
[0043] Step 11, adding lithium salt and tungsten source into deionized water according to the molar ratio Li:W=(1.8-2.2):(0.9-1.1) and stirring to obtain a treatment solution;
[0044] Step 12, adding a positive electrode material containing at least nickel-cobalt-aluminate-lithium-aluminate into the treatment solution, and mixing and stirring at a first specified temperature until the deionized water is completely evaporated to obtain an intermediate product;
[0045] Step 13, drying the intermediate product;
[0046]In step 14, the dried intermediate product is sintered at a second specified temperature in an oxygen atmosphere, and cooled to a greenhouse to obtain a positive electrode material coated with lithium tungstate containing at least lithium nickel cobalt aluminate.
[00...
Embodiment 1
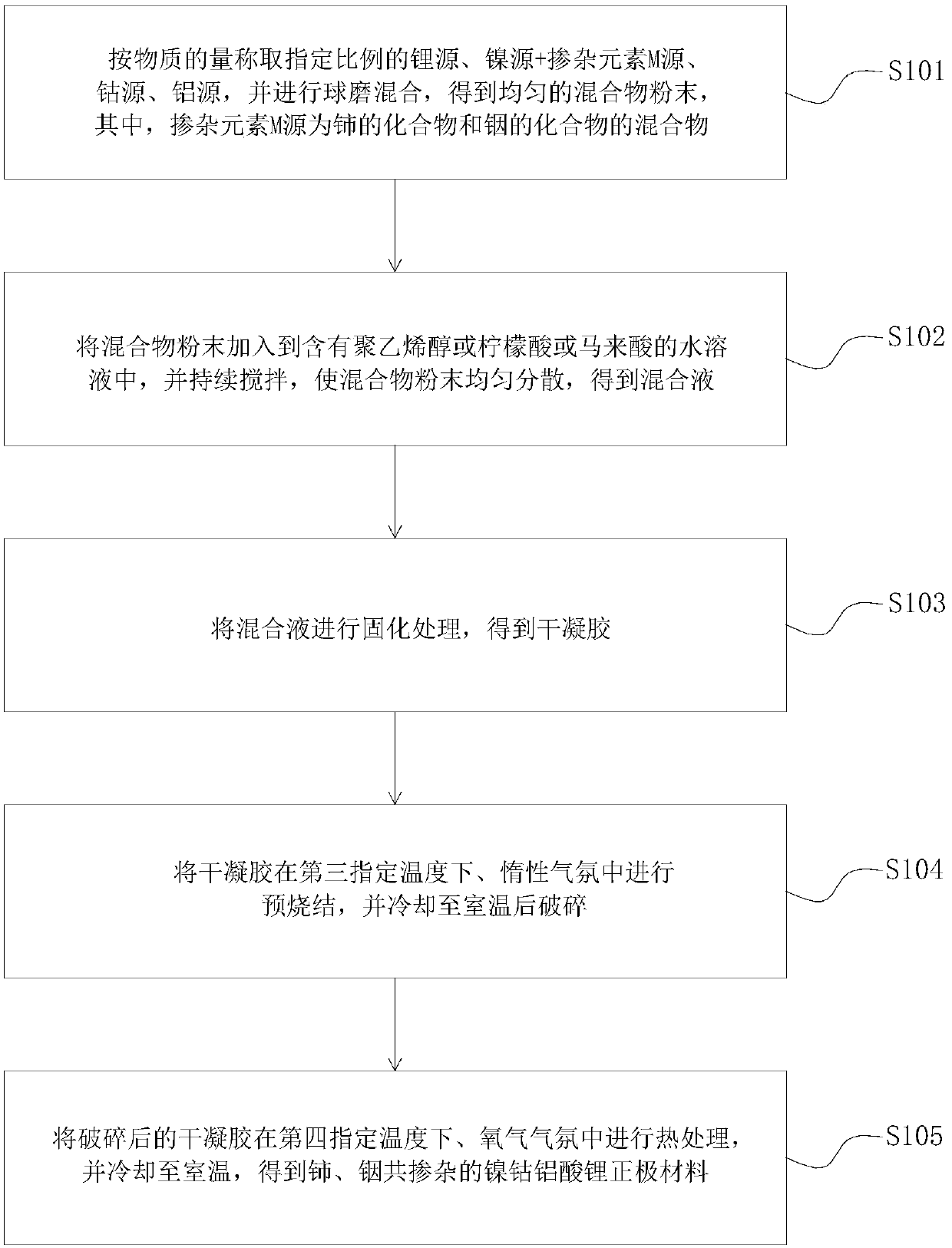
[0071] Weigh lithium sulfate, nickel sulfate, cobalt sulfate, aluminum sulfate, cerium sulfate and indium sulfate according to the ratio of ion molar ratio Li:Ni:Co:Al:Ce:In=1.1:0.78:0.15:0.05:0.01:0.01 In the mixer, ball mill and mix for 1 hour to obtain a uniform mixture powder;
[0072] Add the mixture powder into the aqueous solution containing polyvinyl alcohol, and keep stirring for 30 minutes to obtain the mixed solution;
[0073] Heat the mixed solution to 90°C and keep warm until the solvent in the mixed solution evaporates completely to obtain a wet gel;
[0074] drying the wet gel at 100°C to obtain a dry gel;
[0075] The xerogel was pre-sintered at 600°C in an argon atmosphere for 4 hours, naturally cooled to room temperature and then broken, and the broken xerogel was heat-treated at 800°C in an oxygen atmosphere for 12 hours, and naturally cooled to room temperature to obtain cerium and indium Co-doped nickel-cobalt lithium aluminate cathode material LiNi 0.7...
Embodiment 2
[0081] Weigh lithium acetate, nickel acetate, cobalt acetate, aluminum acetate, cerium acetate and indium acetate according to the ratio of ion molar ratio Li:Ni:Co:Al:Ce:In=1.1:0.79:0.15:0.05:0.005:0.005 In the mixer, ball mill and mix for 1 hour to obtain a uniform mixture powder;
[0082] Add the mixture powder into the aqueous solution containing polyvinyl alcohol, and keep stirring for 30 minutes to obtain the mixed solution;
[0083] Heat the mixed solution to 90°C and keep warm until the solvent in the mixed solution evaporates completely to obtain a wet gel;
[0084] drying the wet gel at 100°C to obtain a dry gel;
[0085]The xerogel was pre-sintered at 600°C in an argon atmosphere for 4 hours, naturally cooled to room temperature and then broken, and the broken xerogel was heat-treated at 750°C in an oxygen atmosphere for 15 hours, and naturally cooled to room temperature to obtain cerium and indium Co-doped nickel-cobalt lithium aluminate cathode material LiNi 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com