Polypeptides having cellulolytic degradation enhancing activity and application thereof
A technology for enhancing activity and cellulose decomposition, applied in the direction of microorganism-based methods, using vectors to introduce foreign genetic material, biochemical equipment and methods, etc., can solve the problem of low activity and achieve the effect of improving utilization efficiency and improving saccharification efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Acquisition of Trichoderma reesei Genome
[0083] (1) Trichoderma reesei was cultured on the bran medium for three days, and then the spores were eluted with physiological saline, according to 1×10 8 Proportionally inserted into the basic medium, 200rpm, 30°C for two days, centrifuged at 4000rpm, collected bacteria, collected in a 1.5ml centrifuge tube;
[0084] (2) Add 500 μl of extraction buffer and 0.1 g of quartz sand to the centrifuge tube, vortex vigorously for 1 min to disperse the bacteria in the extraction buffer, place at 65°C for 20 min; add 500 μl of phenol / chloroform (mixing volume ratio 1:1), vortex vigorously for 30s, centrifuge at 12000rpm for 10min at room temperature, and collect the supernatant;
[0085] (3) Transfer the supernatant to another sterile 1.5ml centrifuge tube, add 0.1 times the volume of 3M NaAc solution (pH4.8) and 0.6 times the volume of isopropanol, mix it upside down, and place it at -20°C for 15 minutes ;12000rpm, 4°C, 10min, disc...
Embodiment 2
[0087] Expression and Purification of Mature Polypeptide LPMO-N in Escherichia coli
[0088] (1) Codon optimization is performed on the mature polypeptide of SEQ ID NO.1 to obtain SEQ ID NO.2, and then gene synthesis is performed. Primers were selected, and the target gene was amplified with KOD FX DNA polymerase from TOYOBO Company. After agarose gel electrophoresis to verify the correct size of the band, the band was cut out, and the gene fragment was recovered with an OMEGA gel extraction kit.
[0089] E. coli-LPMO-N-F:
[0090] GGCCAGTCGAACCACGCAATGCGTCTCGATCCGCAGTGTCTTGCGTCTCTATGCCGAGCACCAAAGTGGCAGCGT, as shown in SEQ ID NO.4;
[0091] E.coli-LPMO-N-R:
[0092] GCACACAGGAAACAGCTATGACCGTCTCGGTTGGCAGTGACTCCGTCTCTTCAGTGATGATGATGATGATGATGCAGC, as shown in SEQ ID NO.5;
[0093] PCR conditions were: 94°C for 5 min; 98°C for 10 s, 58°C for 30 s, 68°C for 1.5 min, 30 cycles; 68°C for 10 min, stored at 4°C to obtain Fragment 1.
[0094] (2) Using the pet21a plasmid of EMD Bio...
Embodiment 3
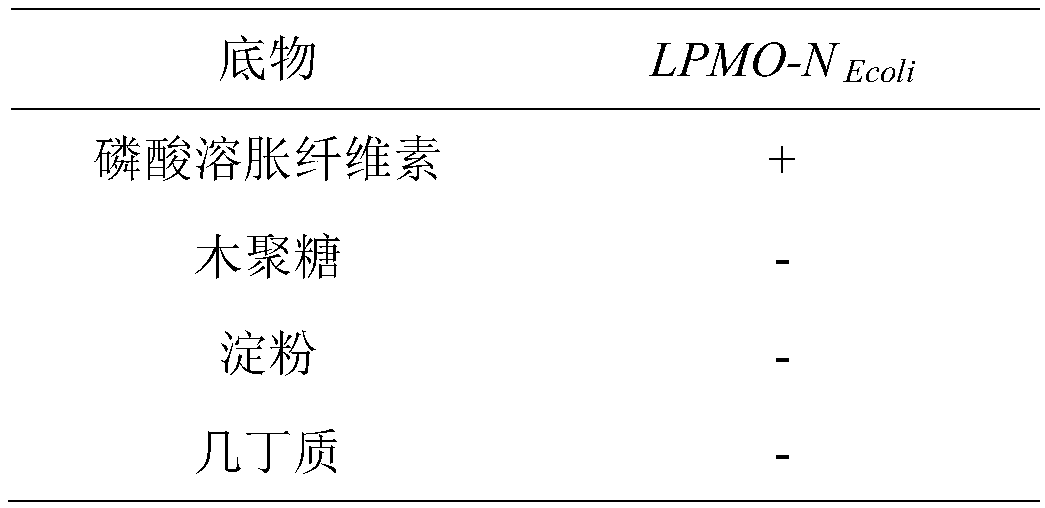
[0106] Degradation experiment of phosphoric acid swollen cellulose:
[0107] Phosphoric acid swollen cellulose (PASC) was prepared from Avicel according to published procedures (see references below).
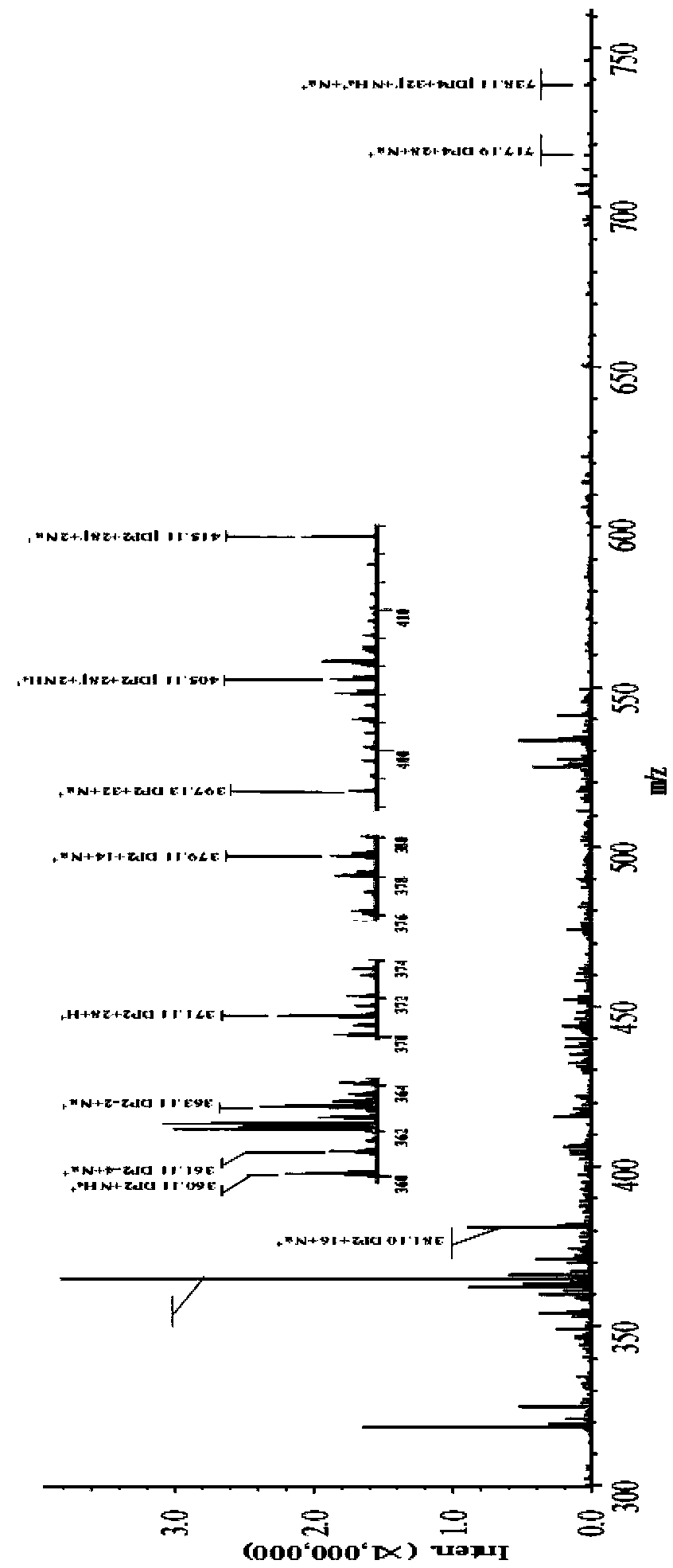
[0108] The reaction was carried out in 100 mM ammonium formate buffer solution, pH 6.0, and the reaction system contained: 4 mM ascorbic acid, 0.5% (w / v) PASC, mixed into the purified LPMO-N in Example 2 Ecoli After 16 hours of incubating the polypeptide at 20°C, the hydrolysis reaction was terminated. Centrifuge at 3000 rpm for 5 min at room temperature. The hydrolysis reaction product in the supernatant was analyzed by MALDI-TOF / TOF MS detection analysis, the mass spectrogram is as follows figure 1 . The product contains the following substances: C-1, C-4 and C-6 oxidized oligosaccharides (oxidized oligosaccharides), aldonic acid (aldonic acid, m / z+16), C4-ketoaldose (ketoaldehyde) or C6-hexodialdose (Adipaldehyde) (m / z-2), minor double C4 and C6 oxidized oligosaccharides...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com