Nucleic acid-drug conjugate, drug delivery system, preparation method and application thereof
A technology of delivery system and conjugate, applied in the field of biomedicine, can solve the problems such as the inability to realize the reasonable and simple construction of a multifunctional drug-carrying system, low degradability, and limitations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0087] The invention provides a nucleic acid-drug conjugate based on nucleic acid phosphorothioate backbone modification, a drug delivery system, their preparation method and application.
[0088] The invention belongs to the field of biomedicine, and specifically discloses a phosphorothioate-modified nucleic acid-based nucleic acid-drug conjugate, a drug delivery system, and a preparation method thereof. The nucleic acid-drug conjugate is formed by grafting the phosphorothioate in the nucleic acid phosphorothioate backbone with the drug molecule modified by the electrophilic reactive group that can react with it. Nucleic acid sequences including functional nucleic acids are selected. In addition, the nucleic acid-drug conjugates can be self-assembled into drug-containing nanocarriers for drug delivery. Compared with the prior art, the nucleic acid backbone of phosphorothioate used in the present invention can be achieved by simple solid-phase synthesis technology, and the gra...
Embodiment 1
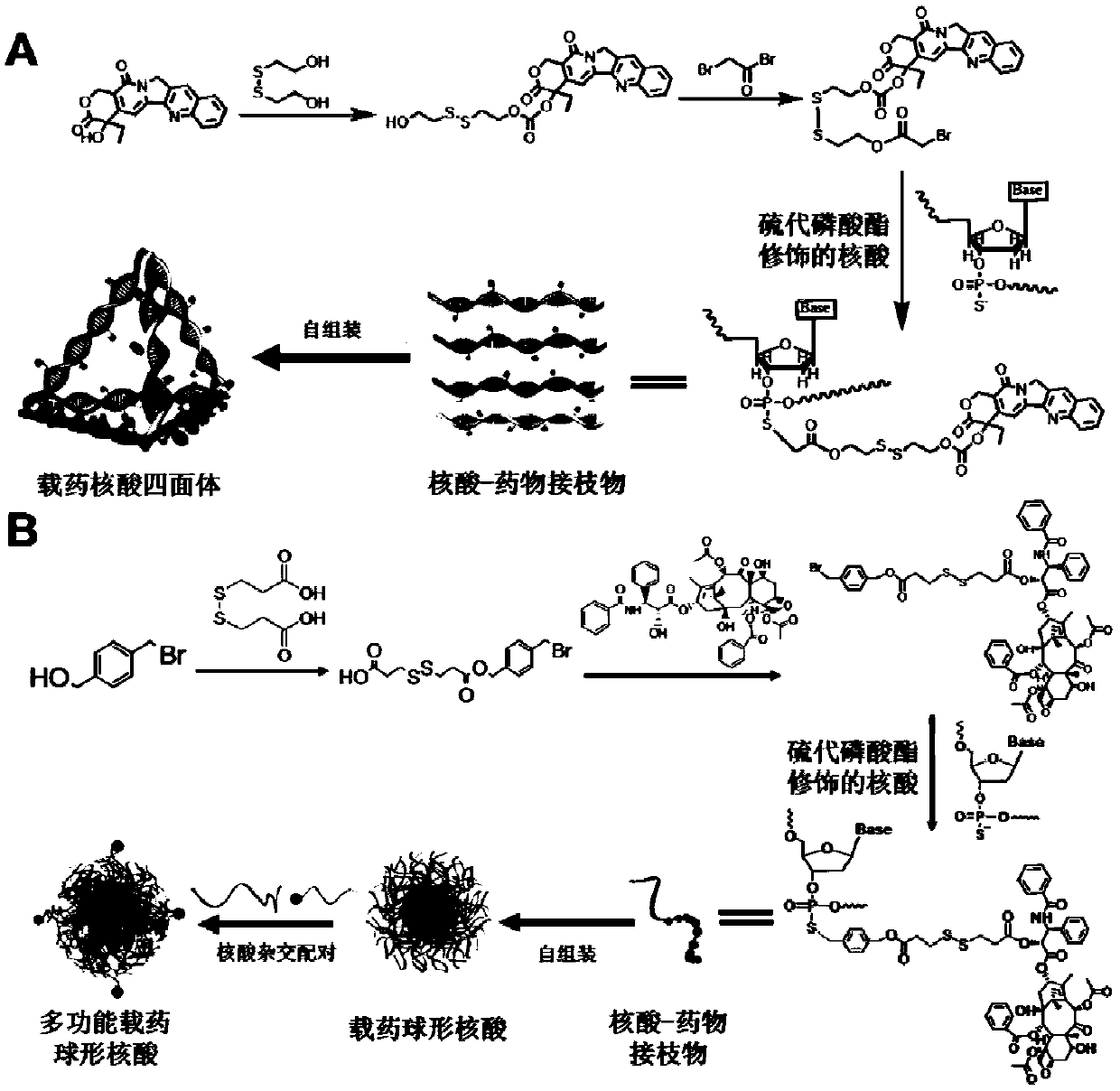
[0092] 1.1 Synthesis of bromocamptothecin prodrug, see steps figure 1 (A), the synthesis is divided into two steps:
[0093] (1), synthesis of redox-sensitive prodrug compound 1: under argon protection, dissolve camptothecin (1g) and triphosgene (313mg) in 150ml of anhydrous dichloromethane, slowly add 4-dimethyl Aminopyridine (DMAP, 1.12g dissolved in 20mL dichloromethane), stirred at room temperature for half an hour, added 2,2'-dithiodiethanol (4.43g), and reacted overnight at room temperature.
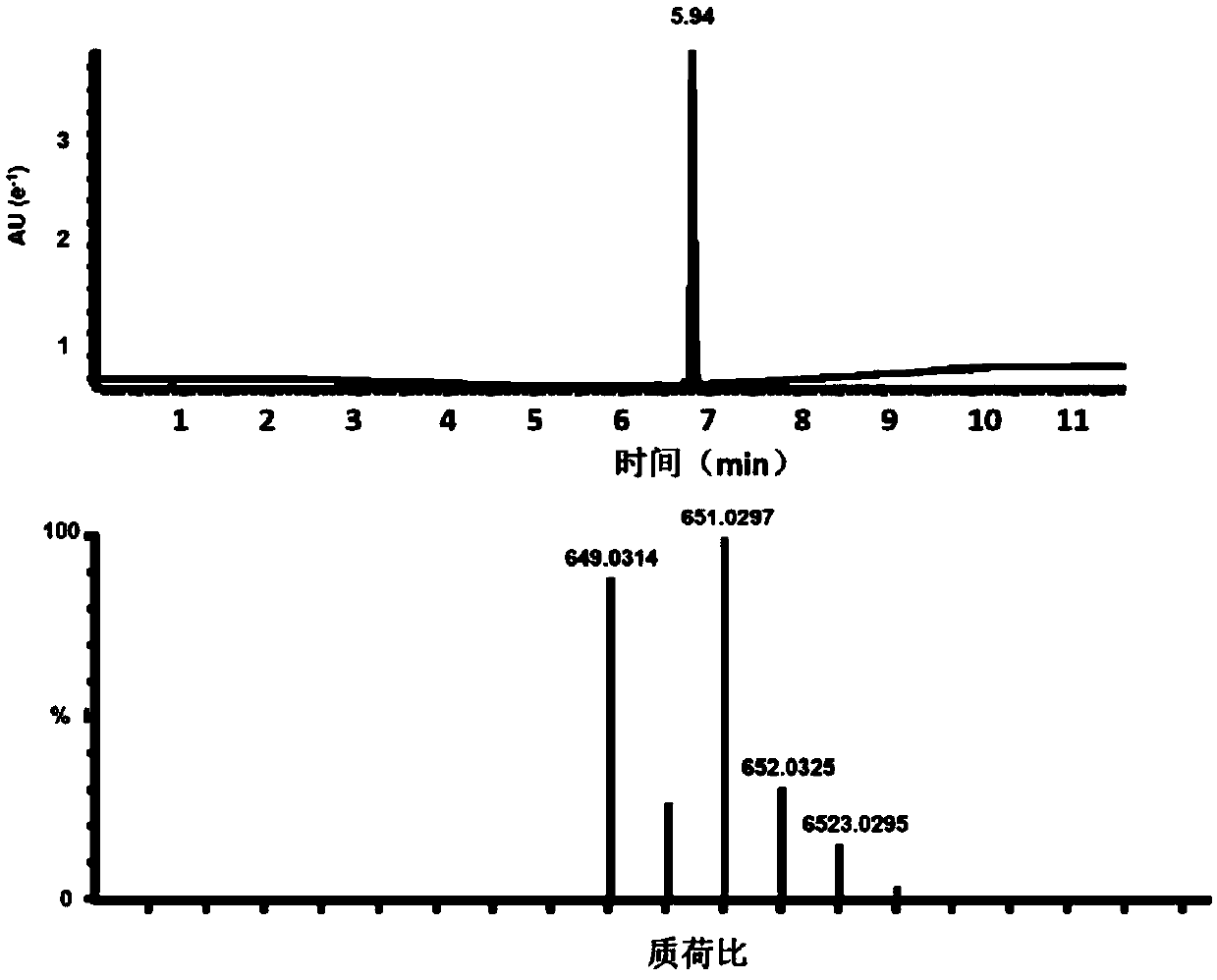
[0094] After the reaction, the mixed solution was washed with 80mL 0.1M HCL solution, separated into layers, discarded the supernatant, washed with HCL solution three times, washed with 80mL saturated NaCl solution, separated into layers, discarded the supernatant, finally washed with 80mL of distilled water, separated into layers , discard the supernatant, and wash with anhydrous MgSO 4 After drying, the crude product was separated and purified by gradient elution of column chro...
example 2
[0107] Synthesis of Example 2 Nucleic Acid-Paclitaxel Graft
[0108] 2.1 Synthesis of benzyl bromide-modified paclitaxel drug (PTX-Bz-Br), the steps are shown in figure 1 (B), its synthesis is also divided into two steps:
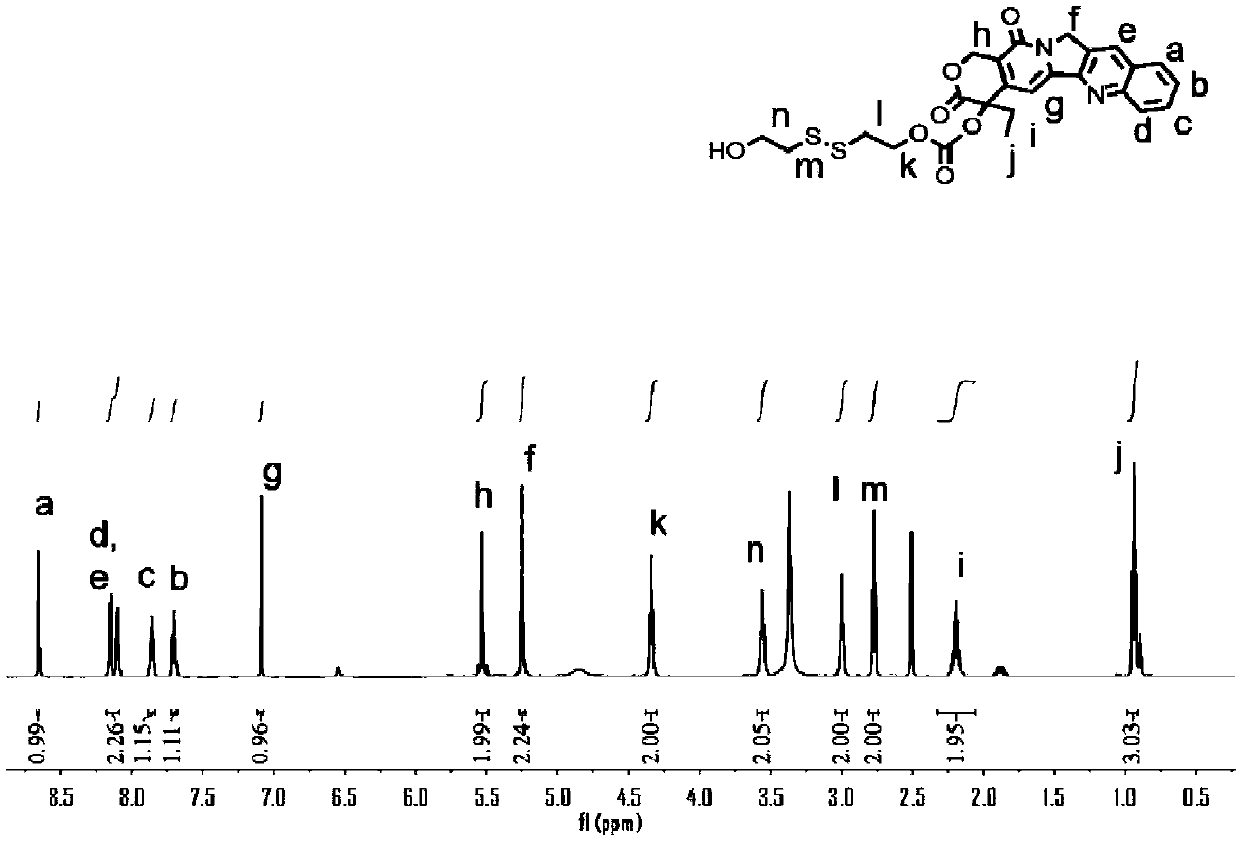
[0109](1) Dissolve 4-bromomethylbenzyl alcohol (500mg, 1 equivalent) and dithiodipropionic acid (DTDP, 2.6g, 5 equivalents) in an ultra-dry mixed solution of methylene chloride and tetrahydrofuran (1 / 1 , v / v); then add DMAP (91mg, 0.3 equivalents), and after stirring for a few minutes, dicyclohexylcarbodiimide (DCC, 615mg, 1.2 equivalents, dissolved in ultra-dry dichloromethane) was added dropwise, and reacted at room temperature After overnight, the solvent was evaporated to dryness using a rotary evaporator, and a benzyl bromide structure (DTPA-Bz-Br) containing a disulfide bond was obtained by silica gel column chromatography, and the eluent was petroleum ether / ethyl acetate. Product NMR map and attribution see Figure 9 .
[0110] (2) Paclitaxel (50...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com