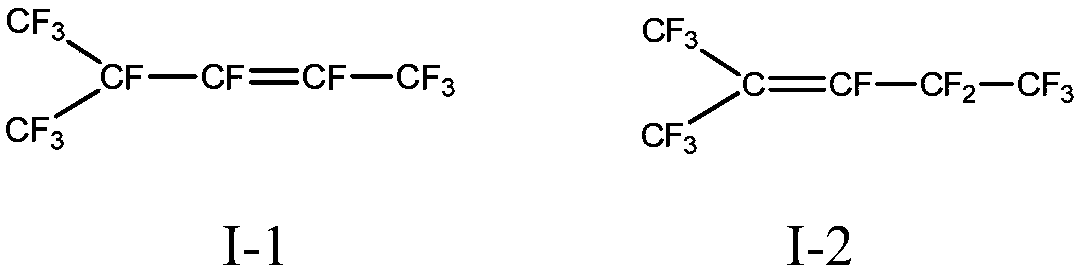
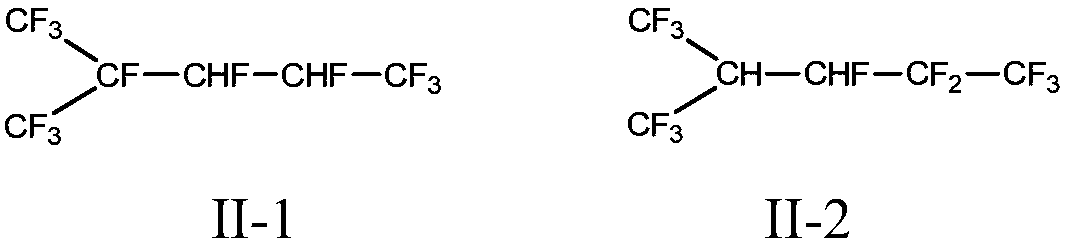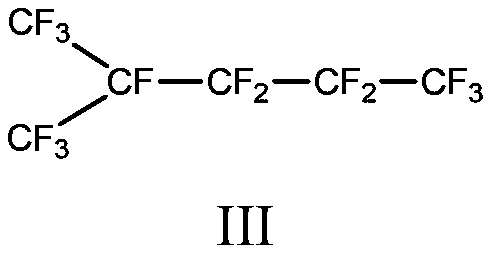Method for preparing branched perfluorohexane
A technology of perfluorohexane and dodecafluorodihydrohexane is applied in the field of preparing branched perfluorohexane, and can solve the problems of undisclosed conversion rate or yield, corrosion of electrolytic cells, pipelines and related equipment, fluorination Reagents are difficult to obtain and other problems, to achieve the best conversion rate of raw materials, high hydrogen replacement reaction efficiency, and improved yield per unit time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of hydrogenation catalyst
[0032] The chloride of Rh, Pd, Ni or Zn is dissolved in the hydrochloric acid aqueous solution according to metal weight ratio as shown in Table 1, and the metal weight percent content is 8%, and the concentration of hydrochloric acid is 11%; After 48 hours, it was drained, dried at a low temperature of 130°C, and calcined at 500°C to obtain the hydrogenation catalyst.
Embodiment 2~8
[0033] Embodiment 2-8: Synthesis of dodecafluorodihydrohexane
[0034] Catalytic hydrogenation of hexafluoropropylene dimer to prepare dodecafluorodihydrohexane. The test was carried out in a fixed-bed reactor. The reactor was a stainless steel tube with an inner diameter of 20mm and a length of 800mm, filled with 150ml of hydrogenation catalyst, and the reaction temperature was 150°C. The operating pressure is 1MPa, and the space velocity of the raw material hexafluoropropylene dimer is 48h -1 , the flow ratio of hydrogen to hexafluoropropylene dimer is 8.
[0035] The synthetic product was analyzed and tested by gas chromatography, and the product was collected by low temperature condensation. The reaction analysis results are shown in Table 1.
[0036]
[0037]
[0038]
Embodiment 9
[0039] Embodiment 9: Synthesis of branched perfluorohexane
[0040] Put 2.8kg of anhydrous hydrofluoric acid in a 5L small electrolytic cell equipped with a -40°C reflux condensing device and necessary auxiliary agent tanks, and remove water by energizing it with nitrogen to maintain a slight positive pressure until the current drops to 0.5mA , voltage 5.5V, then add 14g of dimethyl disulfide, add 280g of dodecafluorodihydrohexane prepared in Example 3, the current gradually rises to 412mA, maintain the voltage at 5.5-5.8V, and the current density of the anode plate is 40mA / cm 2 The continuous electrolysis time is 72 hours, and the temperature of the electrolyte is kept at around 21°C. The layered material at the bottom of the electrolytic cell is recovered, and 255g of perfluorohexane is obtained through steps such as alkali washing, water washing and rectification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com