Method for carrying out liposome modification on compound with free hydroxyl groups
A technology of liposomes and compounds, applied in the direction of steroids, chemical instruments and methods, organic chemistry, etc., can solve the problems of epothilone clinical application limitations, limited tumor treatment applications, and lack of side effects, so as to improve anticancer effect, improve hydrophobicity, and reduce systemic toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Preparation and characterization of liposome N, N'-two-long chain alkane-L-glutamate diamide (LG)
[0049] Add Boc-glutamic acid and two times the equivalent of long-chain amines (octacyl, dodecyl, octadecylamine) to the reaction flask, add dichloromethane to dissolve, then add 1.1 times the equivalent of EDC and HOBt for amide condensation, and then After filtration, washing, and recrystallization, N,N'-di-long-chain alkyl-L-Boc-glutamate diamide was obtained, and then de-Boc protected with trifluoroacetic acid, washed and dried to obtain white powder N,N'-di - long-chain alkyl-L-glutamic acid diamides (LG).
[0050] The reaction steps are as follows:
[0051]
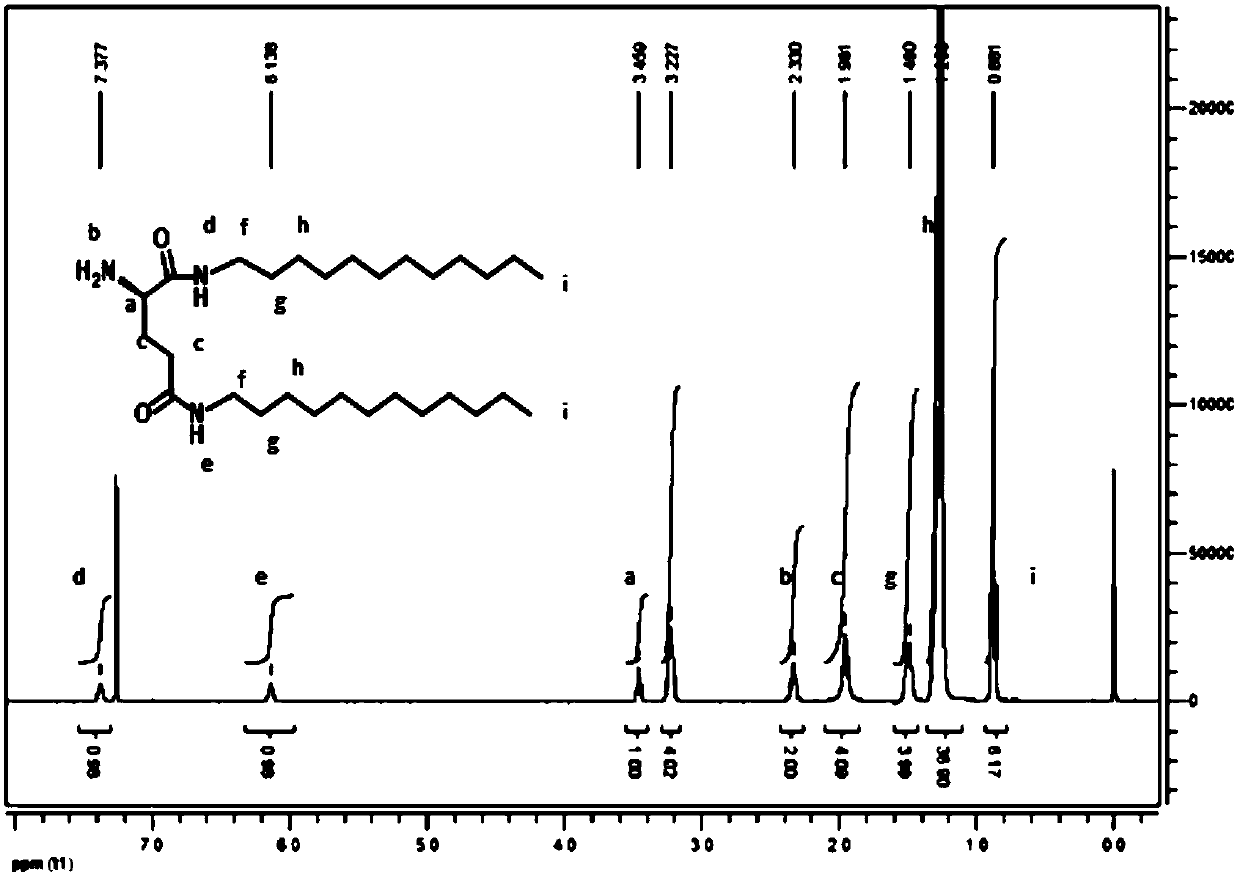
[0052] The prepared LGC12 was characterized by H NMR spectroscopy, the results are as follows figure 1 shown. The peaks in the H NMR spectrum were all assigned successfully, indicating the successful synthesis of LGC12.
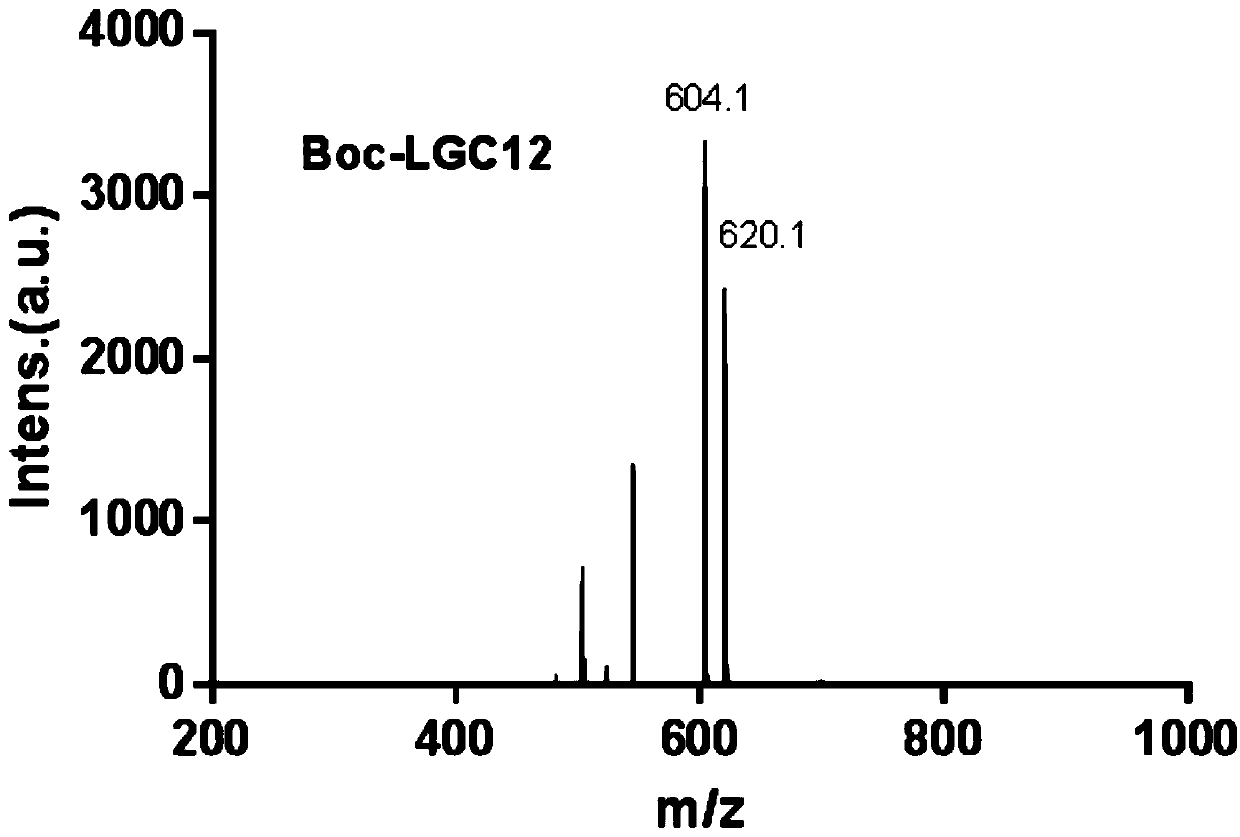
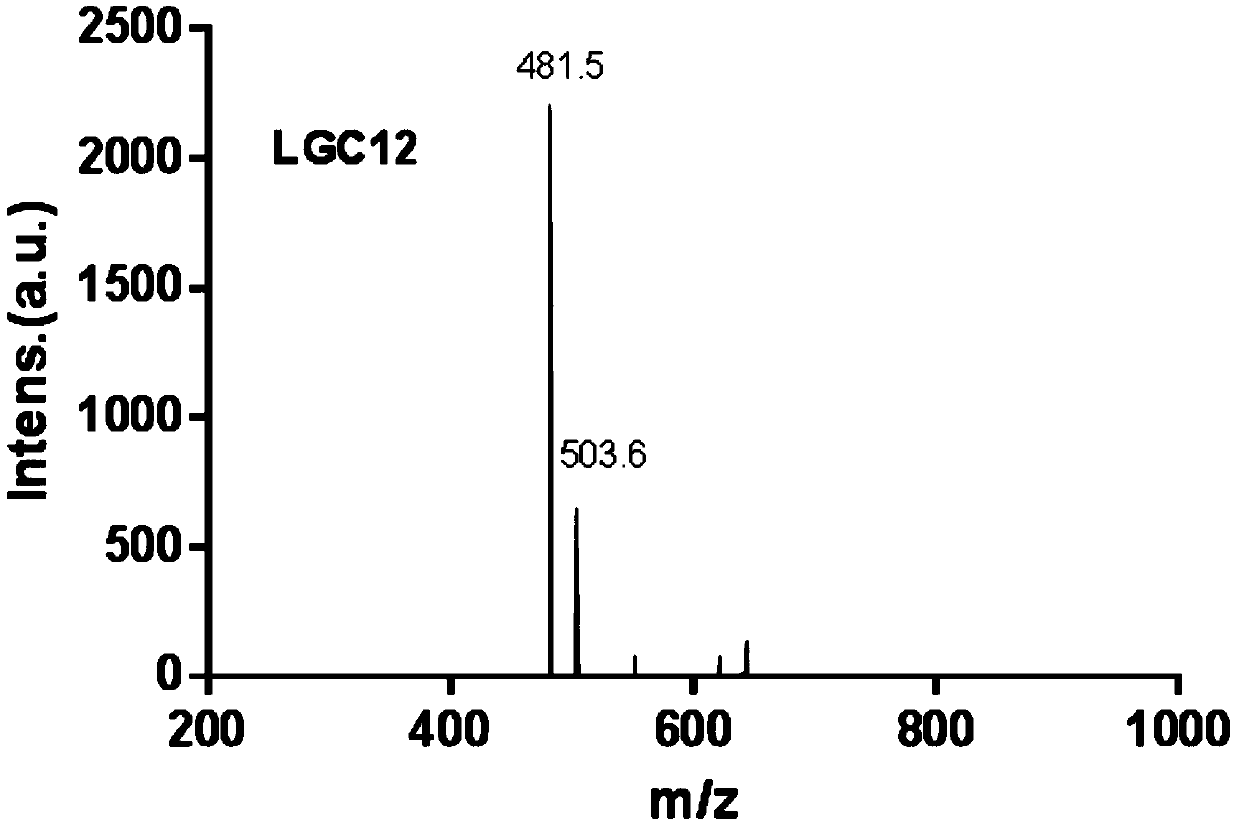
[0053] The prepared LGC12 was characterized by MALDI-TOF-MS, and the res...
Embodiment 2
[0054] Example 2: Preparation method of LGC12 modified Paclitaxel, 10-Hydroxycamptothecin, Irinotecan, Epothilone B
[0055] ①Modify various original drugs with cis-aconitic anhydride (CA) (referred to as R, R = paclitaxel (PTX), 10-hydroxycamptothecin (HCPT), irinotecan (CPT-11), epothilone B (EpoB)), hereafter referred to as CAR, was synthesized by a ring-opening reaction between R and CA using triethylamine as a catalyst. The original drug R (0.4mmol) and CA (0.44mmol) were added into a completely dry flask and dissolved in 20.0 mL of anhydrous DMF, and then 67.0 μL of triethylamine was added. The mixture was placed at room temperature, in the dark, under nitrogen (N 2 ) under the protection of the atmosphere and stirred for 24 hours. Next, the solution was mixed with 200.0 mL of cold ethyl acetate, washed first with a saturated sodium chloride solution of pH 2-3, and then with a saturated sodium chloride solution of pH 7.4. The obtained organic layer was dried over anhy...
Embodiment 3
[0060] Example 3: Structural characterization of LGC12 modified drugs
[0061] 1. Nuclear Magnetic Resonance (NMR)
[0062] Using tetramethylsilane (TMS) as an internal standard, the N,N'-two-dodecane glutamic acid diamide (LGC12) modified PTX prepared in Example 2 was characterized, and deuterated chloroform (CDCl 3 ) as the solvent, adopt 400MHZ nuclear magnetic resonance instrument to its 1 HNMR scans.
[0063] The H NMR spectrum of PTX-LGC12 is as follows image 3 As shown, the peaks in the PTX-LGC12 NMR spectrum were all assigned successfully.
[0064] 2. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS)
[0065] In order to further confirm the compound synthesized in Example 2, its mass spectrum was tested by MALDI-TOF-MS and matrix-selective gentisic acid (DHB).
[0066] The MALDI-TOF-MS mass spectrum of PTX-LGC12 is as follows Figure 4 shown. Figure 4 In 1683.1 is PTX-LGC12+Na + .
[0067] The experimental results of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com