Palladium ion fluorescence probe compound and preparation method and application thereof
A fluorescent probe and compound technology, applied in the field of palladium ion fluorescent probe compound and its preparation, can solve the problems of poor water solubility, harsh test conditions and complicated synthesis steps of palladium ion fluorescent probe, and achieve strong anti-interference ability and convenient Preparation, high sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Preparation of rhodamine amide
[0047] Add ethanol (30mL) and rhodamine B (2g, 4.5mmol) into a 100mL round bottom flask, preheat for 20min, add ethylenediamine (3.0mL, 44.98mmol), reflux at 78-80°C for 15-18h, cool, Ethanol was distilled off at 50°C, the reaction system was extracted with 200mL of dichloromethane and 50mL of water, the organic phase was collected, 5g of desiccant anhydrous sodium sulfate was added, and the filtrate was obtained by filtration. The solvent in the filtrate was evaporated at 50°C to obtain a crude product. Deagent methanol / dichloromethane (volume ratio is V 甲醇 :V 二氯甲烷 =1:100) to carry out column chromatography separation to obtain rhodamine amide.
[0048] (2) Preparation of 6-chloro-8-methyl-9-(naphthalene-1-yl)-9H-purine
[0049]Add 5-amino-4,6-dichloropyrimidine (5g, 30mmol) and 1-naphthylamine (8.584g, 60mmol) into a 100mL round bottom flask, dissolve in ethanol (50mL), and add concentrated hydrochloric acid ( 5mL, 60mmol), ref...
Embodiment 2
[0054] The palladium ion fluorescent probe compound described in Example 1 was used for testing.
[0055] The palladium ion fluorescent probe compound was dissolved in an ethanol solution at a concentration of 20 μM, and the concentration of other metal ions was 100 μM, and tested with a fluorescence spectrometer and an ultraviolet spectrophotometer.
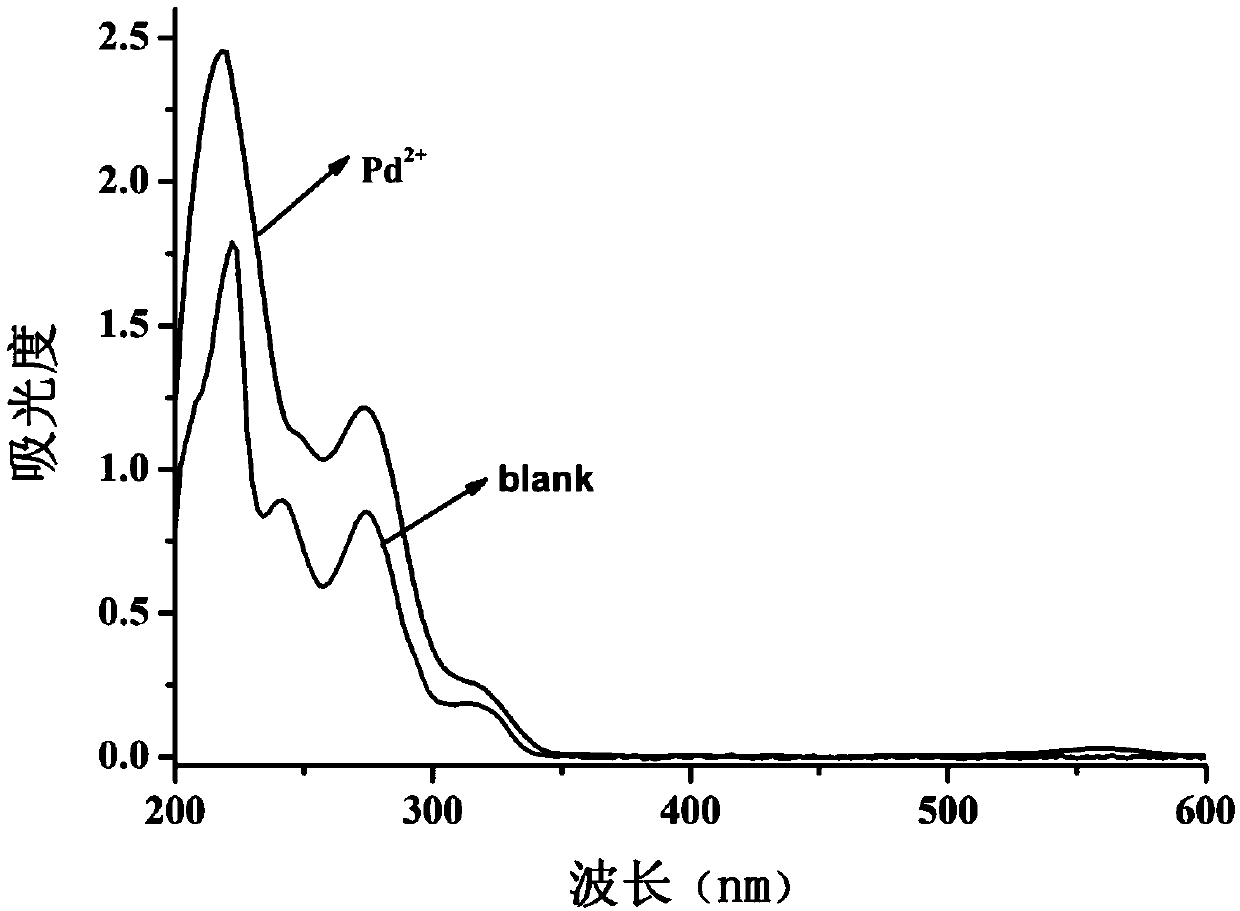
[0056] Such as figure 1 , for the palladium ion fluorescent probe compound of the present invention before and after adding palladium ions, the ultraviolet change spectrum, the probe compound itself has no ultraviolet absorption peak at 550-580nm, after the probe compound molecules are complexed with palladium ions, a new one appears at 562nm the absorption peak.
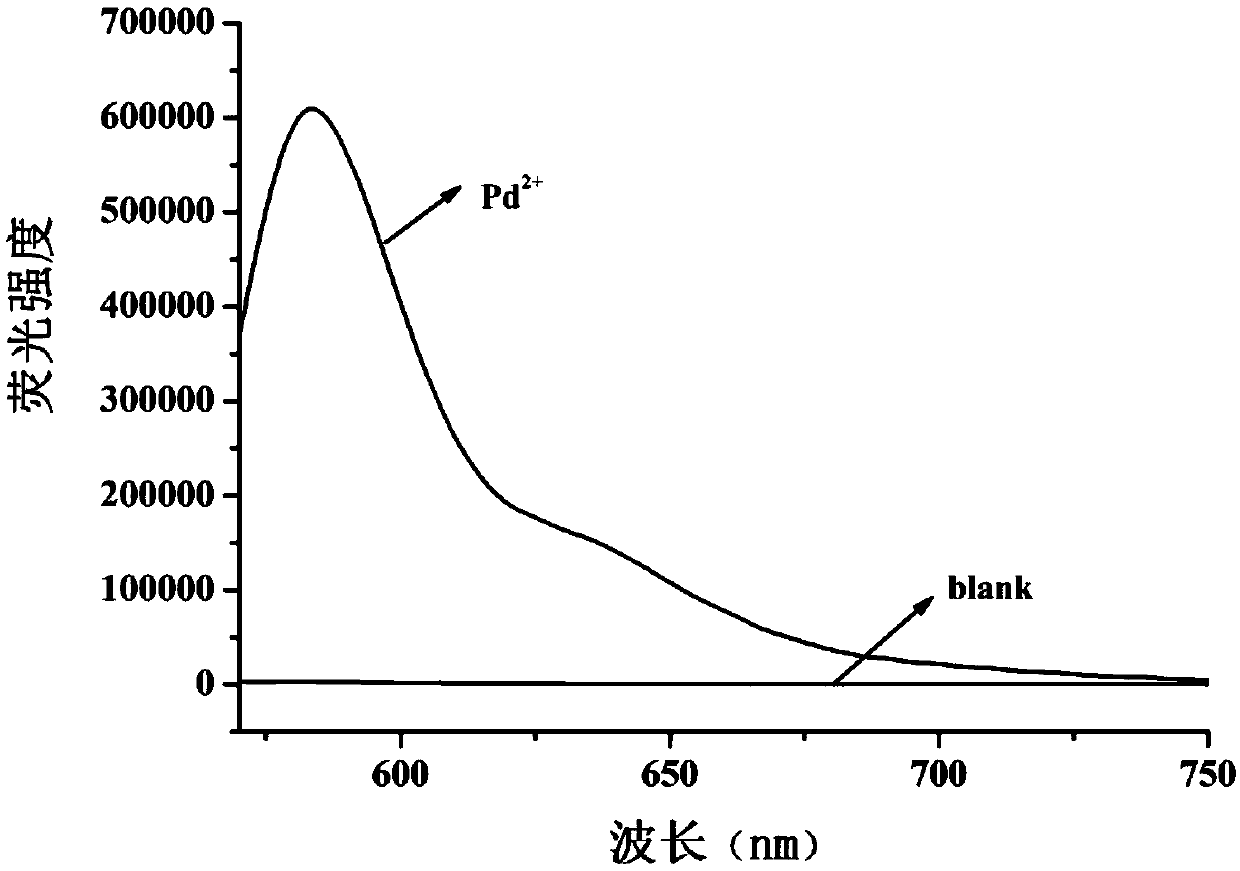
[0057] Such as figure 2 , is the fluorescence change spectrum of the palladium ion fluorescent probe compound of the present invention before and after adding palladium ions, the results show that the probe itself has no absorption in ethanol solution, but with the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com