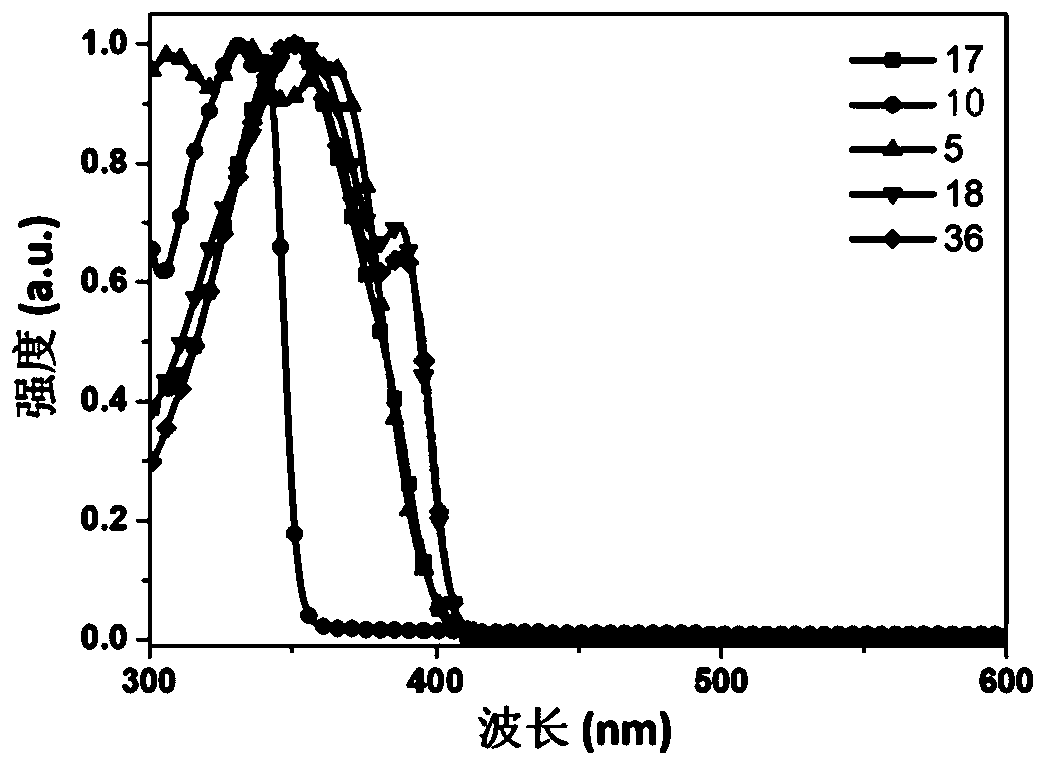
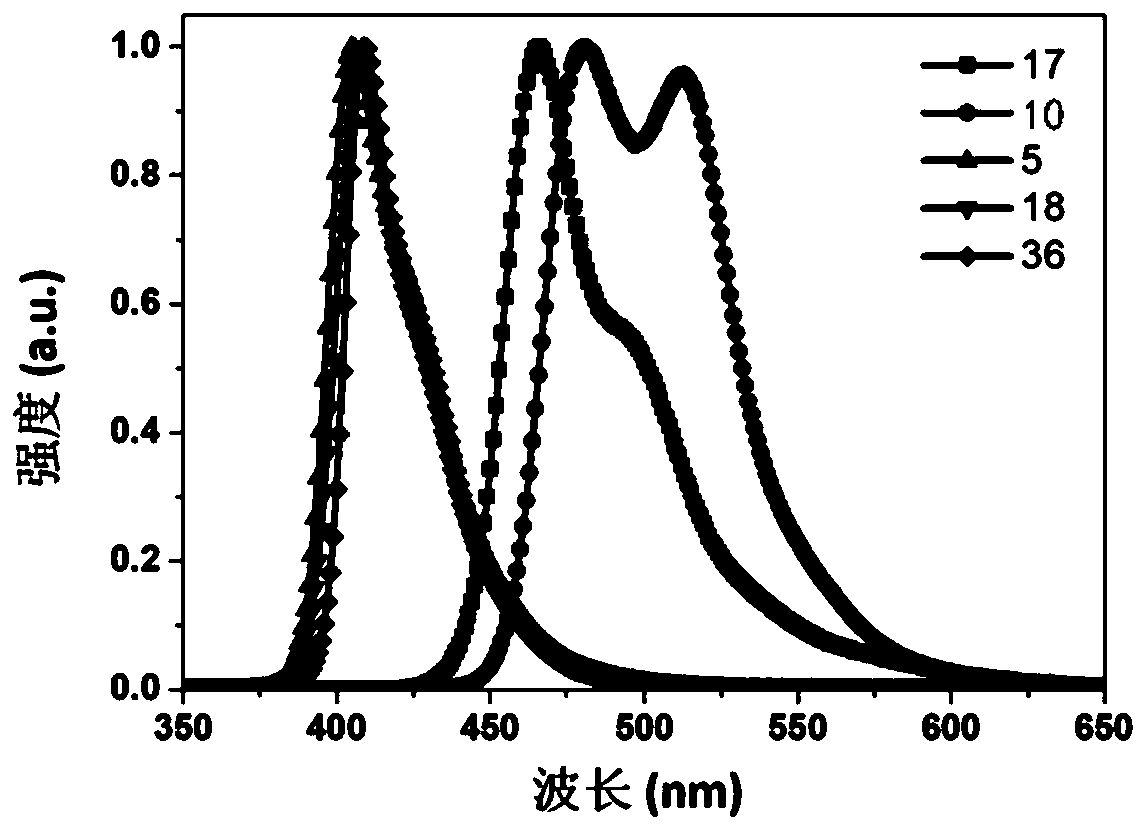
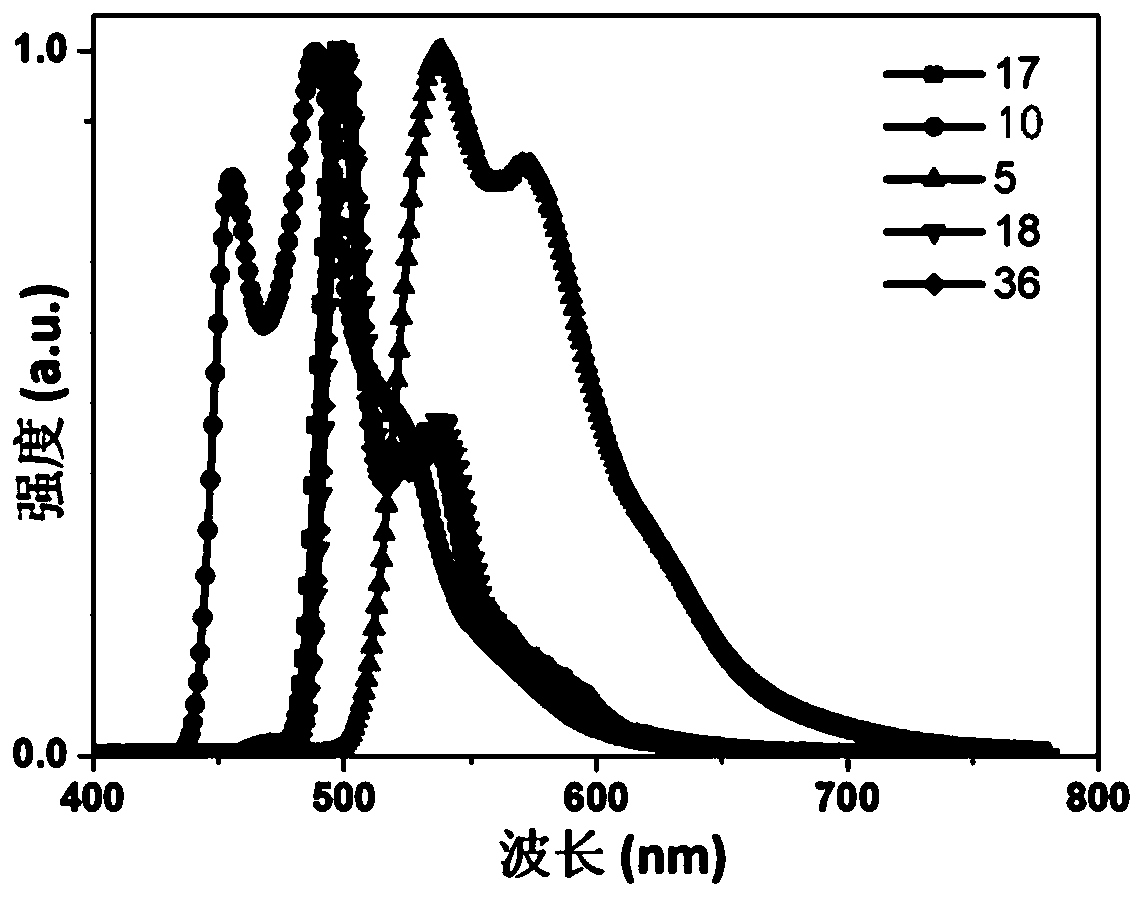
Fluorene derivative and electronic device
A technology of fluorene derivatives and electronic devices, which is applied in the field of organic optoelectronic materials and achieves the effects of simple preparation method, broad industrialization prospect and lower driving voltage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0170] Embodiment 1: the synthesis of compound 10
[0171] (Synthesis of Intermediate 1)
[0172] The synthetic route of intermediate 1 is as follows:
[0173]
[0174] In a 250mL three-necked flask equipped with a thermometer, dropping funnel and mechanical stirring, add 10.0g (36.21mmol) of 2-amino-5,4'-dibromo-benzophenone and 80mL of water, and slowly drop Add 5.0g (50mmol) 98% concentrated sulfuric acid, stir for 30 minutes, cool to 0°C, add dropwise a solution of 1.5g (21mmol) sodium nitrite dissolved in 5mL water, after the dropwise addition is complete, keep this temperature for 30 minutes, slowly Raise the temperature to 60°C. After the bubbles are completely released, cool to room temperature and filter to obtain a light yellow crystalline solid. The solid was separated by column chromatography (350 mesh silica gel, the eluent was petroleum ether:dichloromethane=20:1 (V / V)), the solvent was evaporated, and after drying, 7.50 g of light yellow crystals were obtai...
Embodiment 2
[0184] Embodiment 2: the synthesis of compound 5
[0185] The synthetic route of compound 5 is as follows:
[0186]
[0187] In a 250mL three-necked flask equipped with a reflux condenser, add 10g (28.4mmol) intermediate 2A, 18.69g (85.21mmol) 1-naphthylaminobenzene, 318mg (1.42mmol) palladium acetate, 824mg (2.84mmol) ) tri-tert-butylphosphine tetrafluoroborate, 27.3g (284mmol) sodium tert-butoxide and 100ml anhydrous toluene, heated to reflux, and reacted overnight. Filter after the reaction, spin the filtrate to dryness, and separate by column chromatography (350 mesh silica gel, eluent is petroleum ether: dichloromethane = 4:1 (V / V)), evaporate the solvent, and dry to obtain white Crystallization 15.0g, yield 84%. MS(EI):m / z 628.82[(M+1) + ]. Anal.calcd for C 47 h 36 N 2 (%): C 89.77; H 5.77; N 4.46; found: C 89.50; H 5.98; N 4.52.
Embodiment 3
[0188] Embodiment 3: the synthesis of compound 17
[0189] The synthetic route of compound 17 is as follows:
[0190]
[0191] In a 250mL three-neck flask equipped with a reflux condenser, under nitrogen protection, add 10g (28.4mmol) of intermediate 2A, 29.0g (59.65mmol) of N-(4-(9-phenylcarbazol-3-yl)benzene base) biphenyl-4-amine, 318mg (1.42mmol) palladium acetate, 824mg (2.84mmol) tri-tert-butylphosphine tetrafluoroborate, 5.46g (56.8mmol) sodium tert-butoxide and 100ml anhydrous toluene, heated to reflux , reacted overnight. Filter after the reaction, spin the filtrate to dryness, and separate by column chromatography (350 mesh silica gel, eluent is petroleum ether: dichloromethane = 4:1 (V / V)), evaporate the solvent, and dry to obtain white Crystallization 29.0g, yield 87%. MS(EI):m / z 1163.48[(M+1) + ]. Anal.calcd for C 87 h 62 N 4 (%): C 89.81; H 5.37; N 4.82; found: C89.60; H 5.48; N 4.92.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com