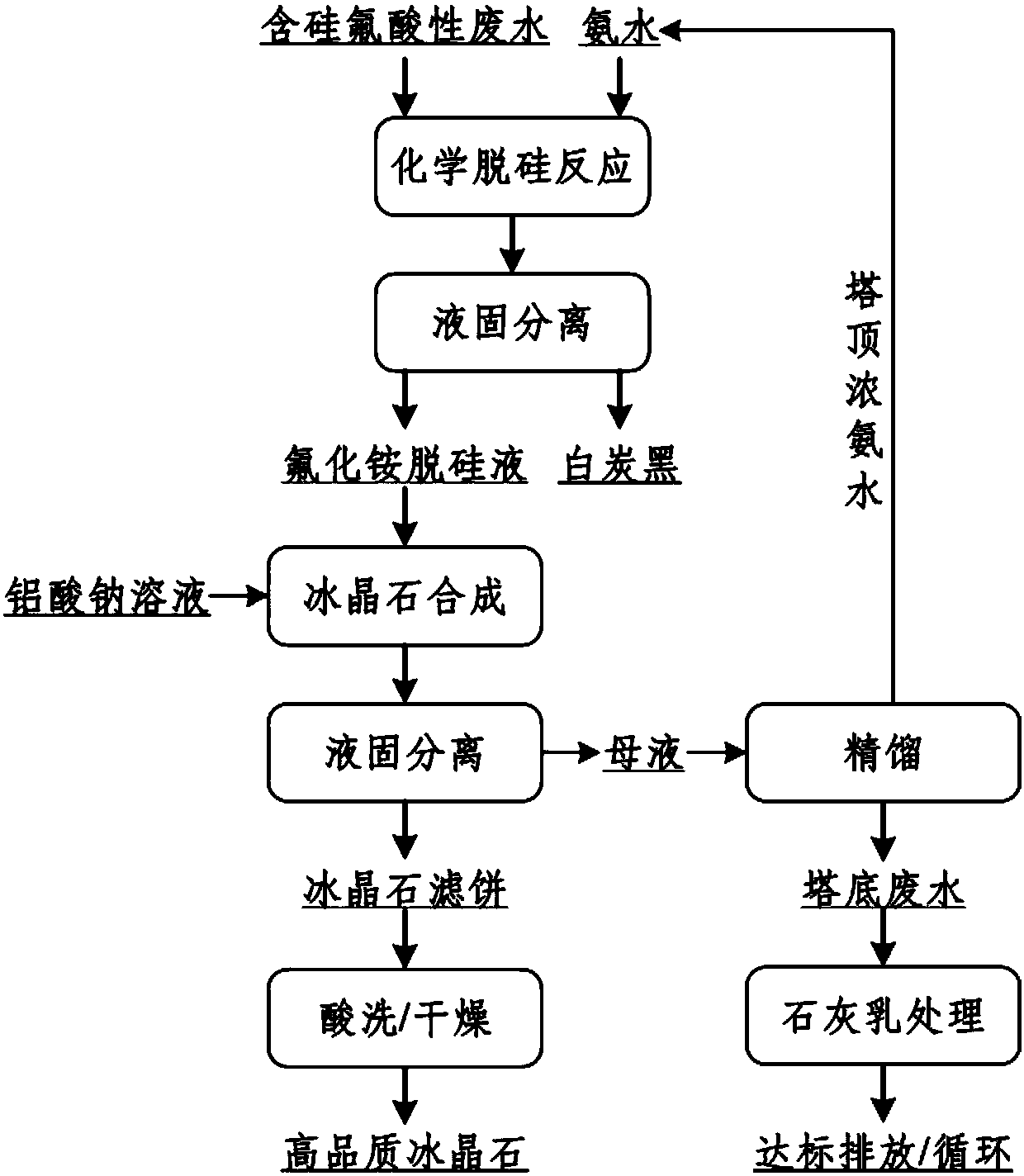
Method for preparing cryolite
A technology of cryolite and wastewater, applied in the direction of aluminum fluoride, silicon dioxide, aluminum halide, etc., can solve the problems of limited quality improvement of silicon content, concentration limit, and unsatisfactory volume of products, so as to facilitate the recycling and reuse of resources , mild conditions, and achieve the effect of resource recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
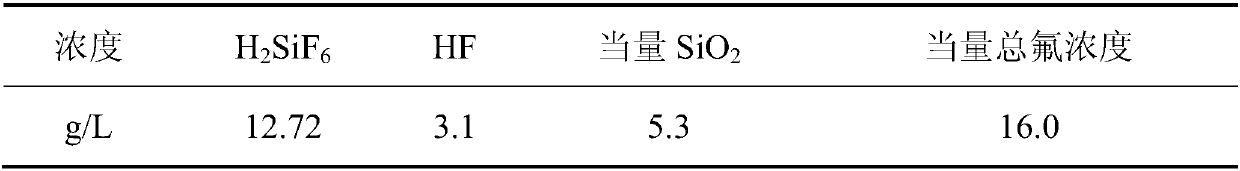
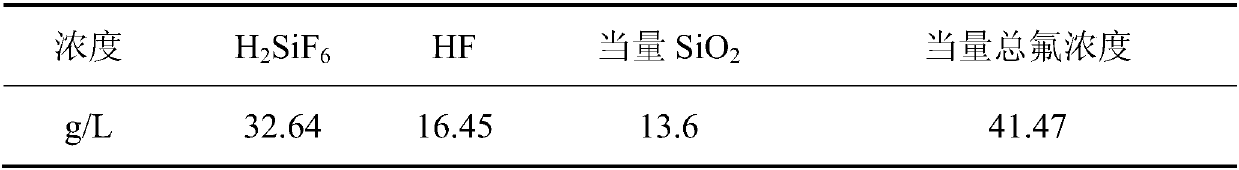
[0070] This example provides a method for preparing cryolite and silica by-products from waste water containing silicon and fluorine. The waste water containing silicon and fluorine used is: acid waste water containing silicon and fluorine obtained from the condensate of a fluidized bed waste gas from an aluminum fluoride plant in Hunan. , the wastewater composition (g / L, the same below) is as described in Table 2:
[0071] Table 2
[0072]
[0073] Specifically include the following steps:
[0074] (1) Mix silicon-fluorine-containing acidic wastewater with the concentrated ammonia water obtained from the rectification top of Example 1, and carry out a chemical desiliconization reaction. The reaction temperature is 90°C, and the desiliconization reaction time is 0.5h. 4 + and F in acidic wastewater containing silicon and fluorine - The mol ratio is 1.05:1, obtains desiliconization reaction slurry;
[0075] Liquid-solid separation and washing of the filter cake to obtain...
Embodiment 3
[0082] Except that the reaction temperature of the chemical desiliconization reaction is 50° C. and the desilication reaction time is 4 h, other preparation methods and conditions are the same as those in Example 1.
[0083] SiO in the cryolite product that present embodiment obtains 2 The mass fraction is 0.086%.
Embodiment 4
[0085] Except that the cryolite synthesis reaction temperature is 100° C. and the synthesis time is 1.5 h, other preparation methods and conditions are the same as in Example 2.
[0086] SiO in the cryolite product that present embodiment obtains 2 The mass fraction is 0.042%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com