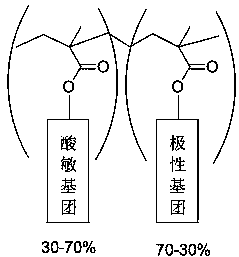
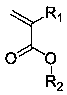
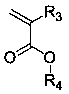
Flexible long-chain multi-onium-salt photoacid generating agent, preparation method thereof and photoresist composition
A photoacid generator and photoresist technology, which is applied in the preparation of sulfonic acid, photosensitive materials for optomechanical equipment, mercaptan preparation, etc., can solve the problems that photoresist resin cannot be used
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Synthesis of bis(2-bromo-2,2-difluoroethoxy)succinate (3)
[0049] Add 41g of 2-bromo-2,2-difluoroethanol (1), 10g of succinic acid (2), 2.92g of p-toluenesulfonic acid and 90mL of Toluene, the mixture was heated to reflux with stirring for 8h. After the reaction was completed, it was cooled, and the reaction solution was washed three times with 50 mL of sodium carbonate aqueous solution and once with 50 mL of saturated brine, and the organic phase was dried with anhydrous sodium sulfate and concentrated to obtain the esterified product bis(2-bromo-2,2-di Fluoroethoxy) succinate (3) was 21 g in total, and the yield was 61%. NMR results: 1 H NMR (CDCl 3 , δ) 2.69, s, 4H; 4.91, t, 4H.
Embodiment 2
[0050] Example 2: Synthesis of bis(2-sodium sulfonate-2,2-difluoroethoxy)succinate (4)
[0051] In a 500 mL round bottom bottle, add 20 g of bis(2-bromo-2,2-difluoroethoxy)succinate (3) and 80 mL of acetonitrile to dissolve, and stir to dissolve. Under the protection of nitrogen, 17.3g of sodium dithionite, 12.5g of sodium bicarbonate and 80mL of aqueous solution were added dropwise, and the reaction solution was heated and stirred at 70°C for 16h after the addition. Then cool, and add an appropriate amount of solid sodium chloride to saturation. The reaction solution was separated into layers, and the aqueous phase was extracted twice with 30 mL of acetonitrile. The organic phases were combined and transferred to a 500mL round bottom bottle, and 100mL of pure water was added. The mixture was added dropwise with 11.2 g of 30% hydrogen peroxide under nitrogen, and then stirred at room temperature for 16 h. After the reaction, the layers were separated, the aqueous phase was ...
Embodiment 3
[0052] Example 3: Synthesis of triphenylsulfonium chloride salt (5)
[0053] Under nitrogen protection, 9.0 g of diphenyl sulfoxide and 60 mL of anhydrous dichloromethane were added into a 250 mL three-necked flask, and the reaction liquid was cooled to below 0°C. Keeping the reaction liquid below 0°C, 14.5 g of trimethylchlorosilane was added dropwise. After the dropwise addition was completed, the temperature was slowly raised to room temperature, and stirring was continued for 1 h. Then the reaction liquid was cooled down to below 0°C again, and at this temperature, 67 mL of 2M phenylmagnesium chloride in tetrahydrofuran solution was added dropwise. After the dropwise addition was completed, the temperature was slowly raised to room temperature, and stirring was continued for 2 h. Then the reaction solution was quenched with a small amount of water, and 75 mL of 0.2N aqueous hydrochloric acid was added. After the mixture was washed twice with 30 mL of diethyl ether, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com