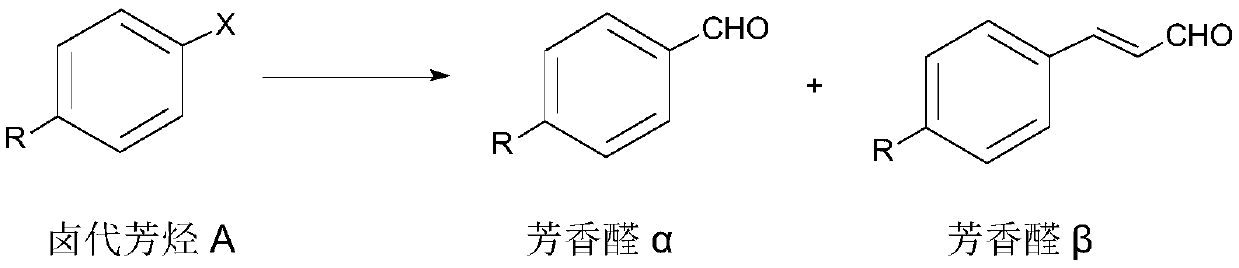
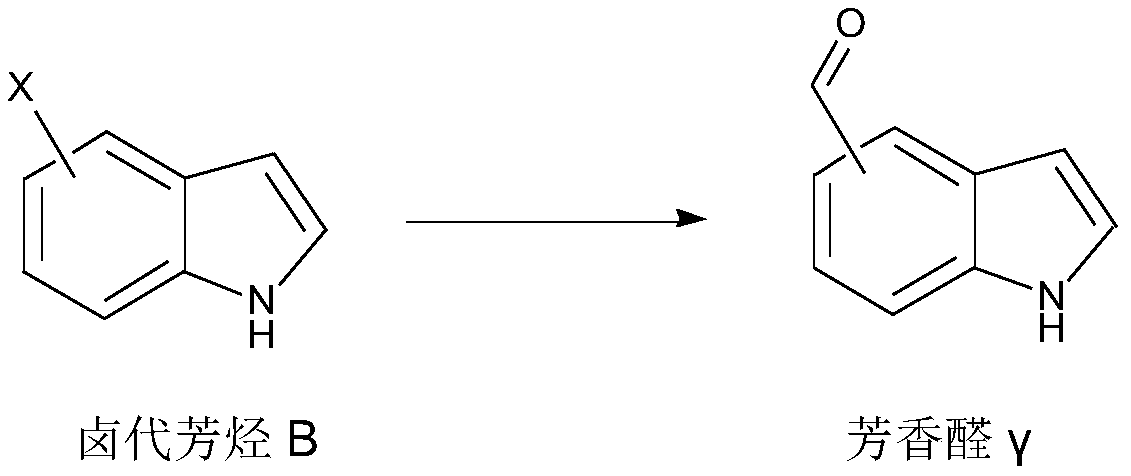
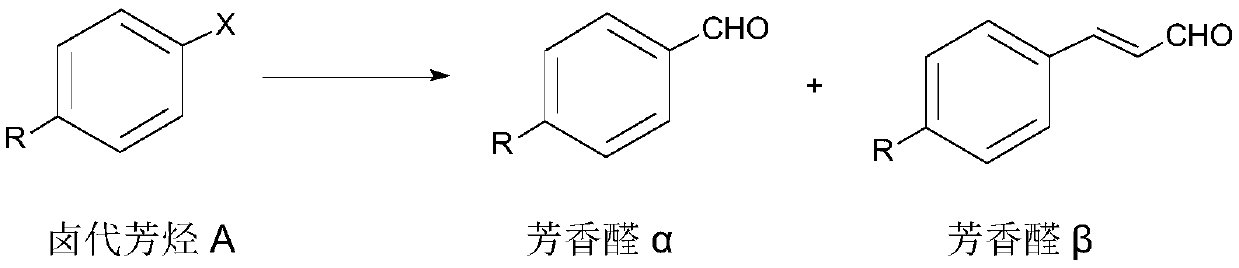
Aromatic aldehyde synthesis method
A synthesis method and technology for aromatic aldehydes, which are applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve the problems of high reaction pressure and harsh conditions, and achieve easy yield, easy product and fast reaction rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] In a preferred embodiment, in order to increase the reaction rate, the above synthesis method further includes the step of adding a free radical initiator.
[0047] In a further preferred embodiment, the free radical initiator is tert-butyl peroxybenzoate.
[0048] According to a preferred embodiment of the second aspect of the present invention, a synthetic method of aromatic aldehyde has the following synthetic route:
[0049]
[0050] Wherein, X is I or Br;
[0051] The synthetic method comprises the following steps:
[0052] Add halogenated aromatic hydrocarbon B, glyoxylic acid, palladium acetate, n-butyl bis(1-adamantyl) phosphine, organic base and polar aprotic solvent into the reaction vessel, heat the reaction, and post-treatment to obtain the aromatic aldehyde γ .
[0053] In a preferred embodiment, in order to increase the reaction rate, the above synthesis method further includes the step of adding a free radical initiator.
[0054] In a further prefe...
Embodiment 1
[0061] Catalytic Synthesis of Benzaldehyde and Cinnamaldehyde
[0062]
[0063] Add iodobenzene (1.02g, 5mmol), palladium acetate (56.12mg, 0.25mmol), triphenylphosphine (262.3mg, 1mmol), DMF 5ml, glyoxylic acid (1.85g, 25mmol), triethylamine to the flask (2.77g, 27.5mmol), tert-butyl peroxybenzoate (48.5mg, 0.25mmol), sealed, heated at 110°C, and reacted for 3 hours. Cool to room temperature, add 150ml of water, extract with dichloromethane 3×100ml, combine the organic phases, wash once with saturated sodium chloride solution, dry over anhydrous sodium sulfate, evaporate the organic solvent under reduced pressure, and purify by column chromatography (the mobile phase is ethyl acetate Esters: petroleum ether = 20:1). 159 mg of benzaldehyde was obtained, with a yield of 30%, and 218 mg of cinnamaldehyde, with a yield of 33%.
[0064] Wherein, the product is characterized as follows:
[0065] Benzaldehyde: 1 H NMR (400MHz, CDCl 3 )δ9.95(s,1H),7.44-7.82(m,5H);
[0066] C...
Embodiment 2
[0068] Catalytic Synthesis of p-methoxybenzaldehyde and p-methoxycinnamaldehyde
[0069]
[0070] Add p-methoxyiodobenzene (1.17g, 5mmol), palladium acetate (56.12mg, 0.25mmol), triphenylphosphine (262.3mg, 1mmol), DMF 5ml, glyoxylic acid (1.85g, 25mmol) into the flask , DBU (4.18g, 27.5mmol), tert-butyl peroxybenzoate (48.5mg, 0.25mmol), sealed, heated at 110°C, and reacted for 3 hours. Cool to room temperature, add 150ml of water, extract with dichloromethane 3×10ml, wash the organic phase once with saturated sodium chloride solution, dry over anhydrous sodium sulfate, evaporate the organic solvent to dryness under reduced pressure, and purify by column chromatography (mobile phase is ethyl acetate: Petroleum ether=15:1). 149.6 mg of p-methoxybenzaldehyde was obtained, with a yield of 22%, and 89 mg of p-methoxycinnamaldehyde, with a yield of 11%.
[0071] Wherein, the product is characterized as follows:
[0072] p-Methoxybenzaldehyde: 1 H NMR (400MHz, CDCl 3 )δ9.89...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com