Preparation method of cage type octaphenylsilsesquioxane(OPS)
A technology of octaphenylsilane and sesquioxane, which is applied in the field of organic-inorganic hybrid materials, can solve the problems of complex process, long cycle, difficult separation, etc., and achieve good product stability, short reaction cycle, and good repeatability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The invention provides a preparation method of cage-type octaphenylsilsesquioxane, which comprises the following steps: mixing phenylsilane, an organic solvent, a basic catalyst and water, performing hydrolysis and polycondensation reaction, and obtaining cage-type octaphenylsilsesquioxane Silsesquioxane.
[0026] In the present invention, unless otherwise specified, all raw material components are commercially available products well known to those skilled in the art.
[0027] In the present invention, the phenylsilane preferably includes phenyltrichlorosilane, phenyltrimethoxysilane or phenyltriethoxysilane.
[0028] In the present invention, the basic catalyst preferably includes potassium hydroxide, lithium hydroxide, sodium hydroxide, potassium carbonate, sodium carbonate, lithium carbonate, sodium methoxide, sodium ethoxide, tetramethylammonium hydroxide and tetraethylhydrogen One or more of ammonium oxides, more preferably potassium hydroxide, lithium hydroxide,...
Embodiment 1
[0039] Add 19.84g of phenyltrimethoxysilane and 200mL of isopropanol into a 500mL three-necked flask equipped with a reflux condenser, a constant pressure dropping funnel, a temperature control device and a magnetic stirrer, and mix evenly at 25°C and 100rpm. Raise the temperature to 85°C, then dissolve 0.3g KOH in 10mL distilled water to obtain a KOH solution, slowly add the KOH solution to the obtained system dropwise at a rate of 0.3mL / min, start timing after the dropwise addition, and react for 48 hours to obtain a white reaction solution ;
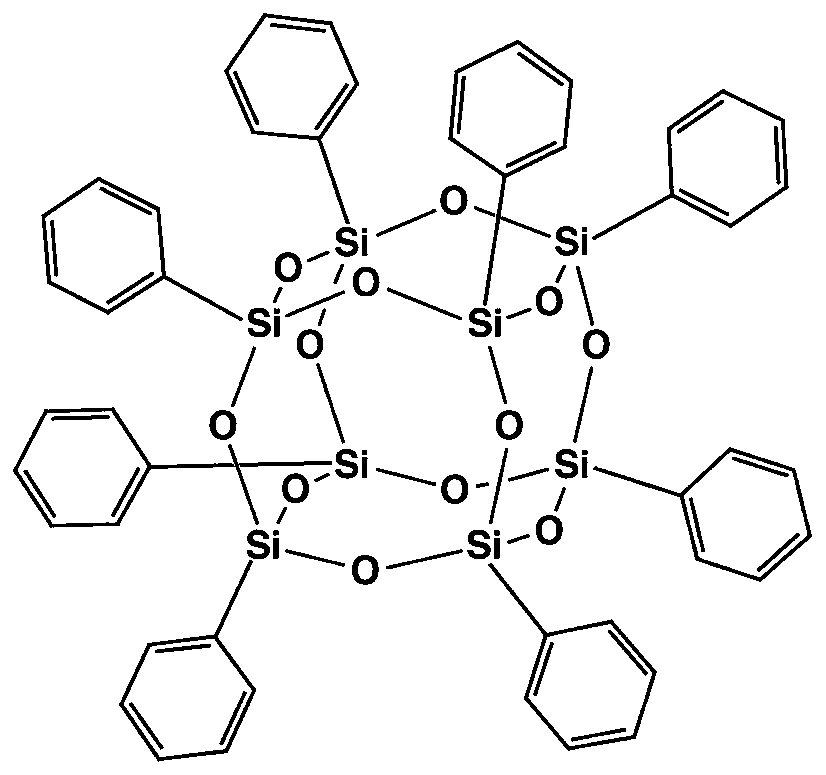
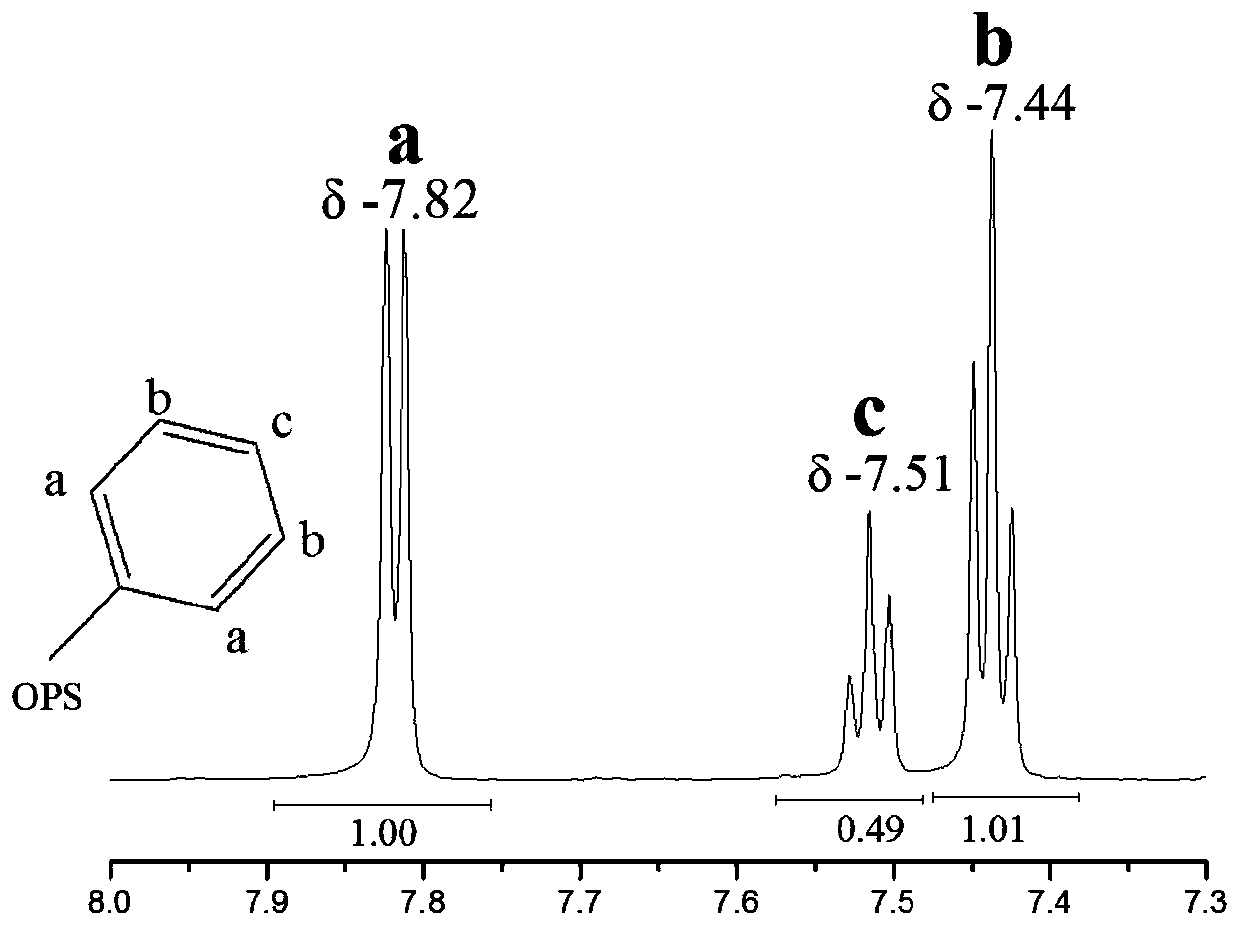
[0040] Suction-filter the obtained white reaction liquid, wash alternately with distilled water and absolute ethanol three times to obtain a filter cake, and dry the filter cake in a vacuum oven at 80° C. for 10 h to obtain cage-type octaphenylsilsesquioxane. The productive rate is 95.3% based on the amount of phenylsilane, and the purity is 99.8%. The structure diagram is as follows: figure 1 shown.
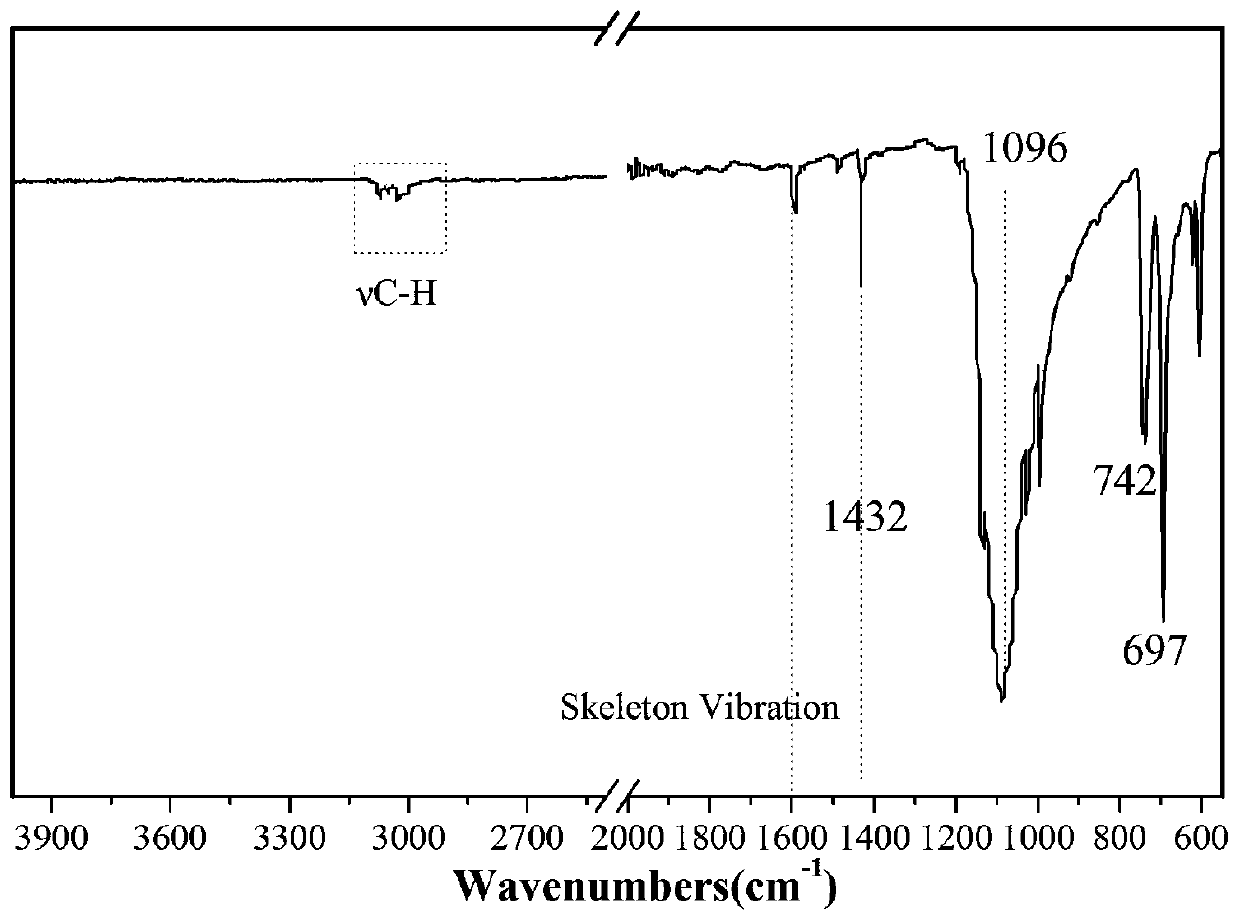
[0041] figure 2 It is the FT...
Embodiment 2
[0047] Add 21.16g of phenyltrichlorosilane and 100mL of dichloromethane into a 500mL three-neck flask equipped with a reflux condenser, a constant pressure dropping funnel, a temperature control device, and a magnetic stirrer, mix well at 25°C and 150rpm, and heat up to 45°C, then dissolve 0.5g of tetraethylammonium hydroxide in 10mL of distilled water to obtain a tetraethylammonium hydroxide solution, and slowly drop the tetraethylammonium hydroxide solution into the resulting system at a rate of 0.3mL / min , start timing after the dropwise addition, and react for 24 hours to obtain a white reaction solution;
[0048] Suction-filter the resulting white reaction solution, wash alternately with distilled water and absolute ethanol three times to obtain a filter cake, and dry the filter cake in a vacuum oven at 80°C for 10 hours to obtain cage-type octaphenylsilsesquioxane. The yield is 96.1%, the purity is 99.8%, and the molecular weight is 1033.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com