Application of geniposide in alleviating side effects of glucocorticoid
A technology of glucocorticoids and side effects, which is applied in the application field of geniposide in alleviating the side effects of glucocorticoids, and can solve problems such as frequent administration, limited applicable population, and high treatment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 MC3T3-E1 cell proliferation experiment
[0045] 1.1 Drugs and reagents
[0046] MC3T3-E1 cell line (Cell Bank, Chinese Academy of Sciences), α-MEM medium (without Vc) (GIBCO, USA), Fetal bovine serum (FBS), dexamethasone (Dex) (Sigma, USA) ), ascorbic acid (vitamin C, Vc) (US Sigma), β-sodium glycerophosphate (US Sigma) NBT / BCIP mixed reagent (US Sigma), alizarin red, CCK-8 (Cell Counting Kit-8) (Japanese colleagues ), alkaline phosphatase activity detection kit (Beiyuntian Biotechnology Co., Ltd.), BCA protein concentration assay kit (Beiyuntian Biotechnology Co., Ltd.), absolute ethanol, geniposide (Chengdu Best Reagent Co., Ltd.).
[0047] 1.2 MC3T3-E1 cell culture
[0048] Use α-MEM medium (without Vc) containing 10% fetal bovine serum to culture MC3T3-E1 cells in a 5% CO2 incubator at 37°C, change the medium every 3 days, and when the cells grow to about 90% of the bottom of the bottle , digested with 0.25% trypsin, centrifuged, collected cells, and ca...
Embodiment 2
[0052] Example 2 Cell alkaline phosphatase staining and activity detection
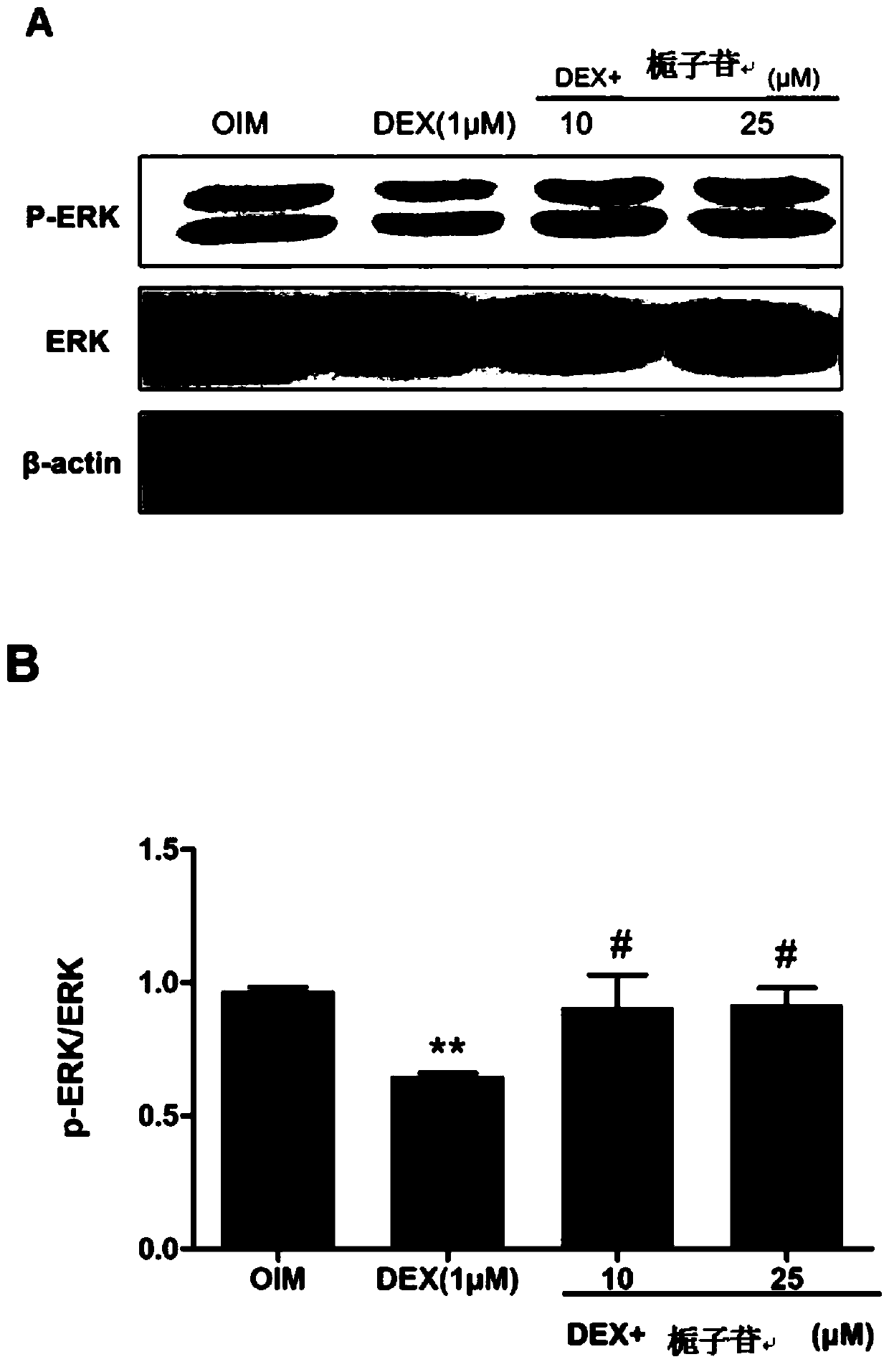
[0053] Alkaline phosphatase (ALP) is a marker glycoprotein in the early differentiation of osteoblasts, and its activity is closely related to the activity of osteogenic differentiation. The early stages of osteogenic differentiation and functional maturation can be used as one of the indicators to evaluate bone formation and bone turnover. However, under the action of superphysiological glucocorticoids, the activity of alkaline phosphatase in the process of osteogenic differentiation in vitro will be inhibited and affect the osteogenic differentiation of cells. In this experiment, alkaline phosphatase staining and quantitative detection of alkaline phosphatase activity were used to study the effect of geniposide on the inhibition of alkaline phosphatase activity in MC3T3-E1 cells induced by dexamethasone.
[0054] Cell digestion and inoculation: Take well-grown MC3T3-E1, digest and count, and use 2×...
Embodiment 3
[0057] Example 3 Mineralized nodule staining and quantitative detection
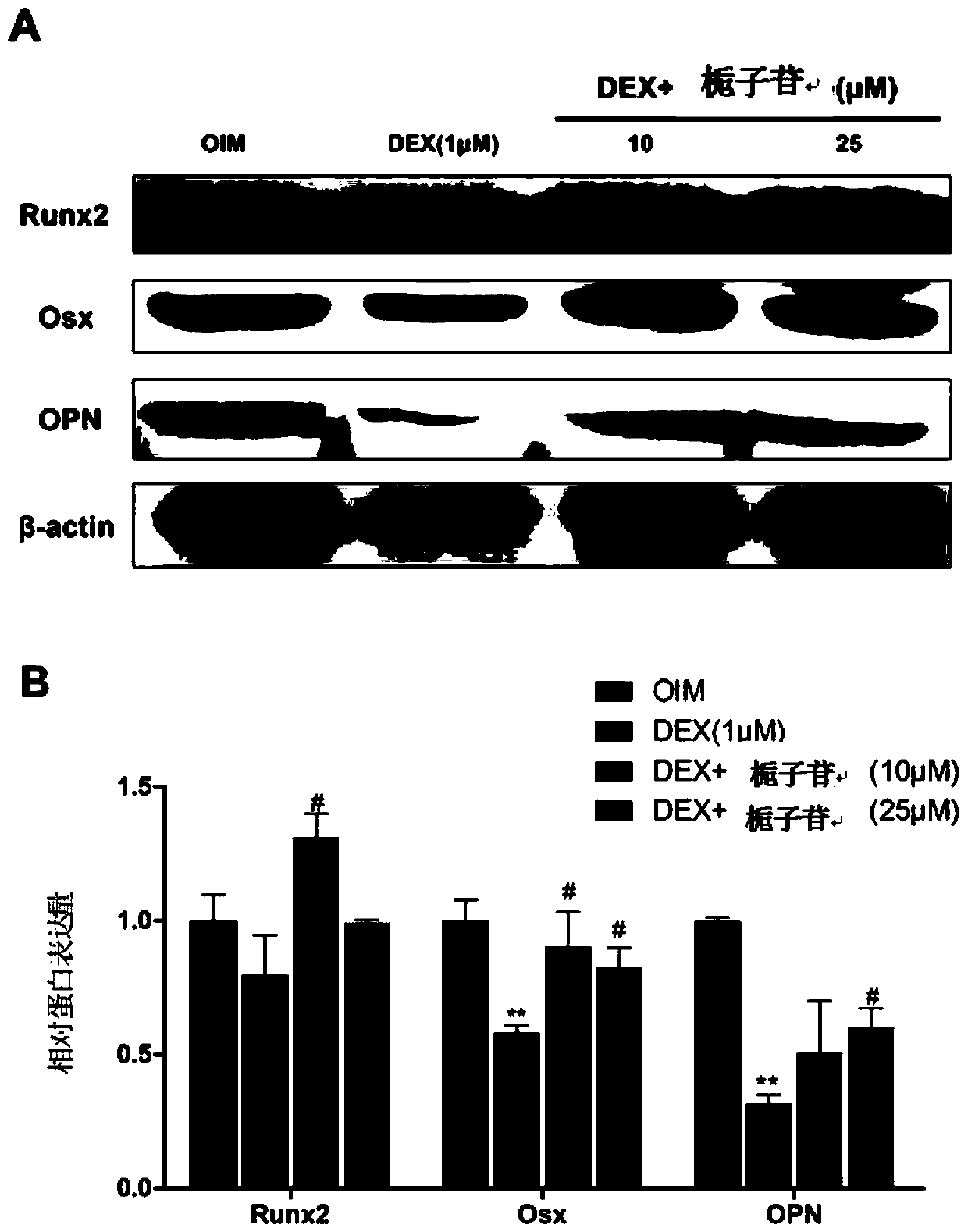
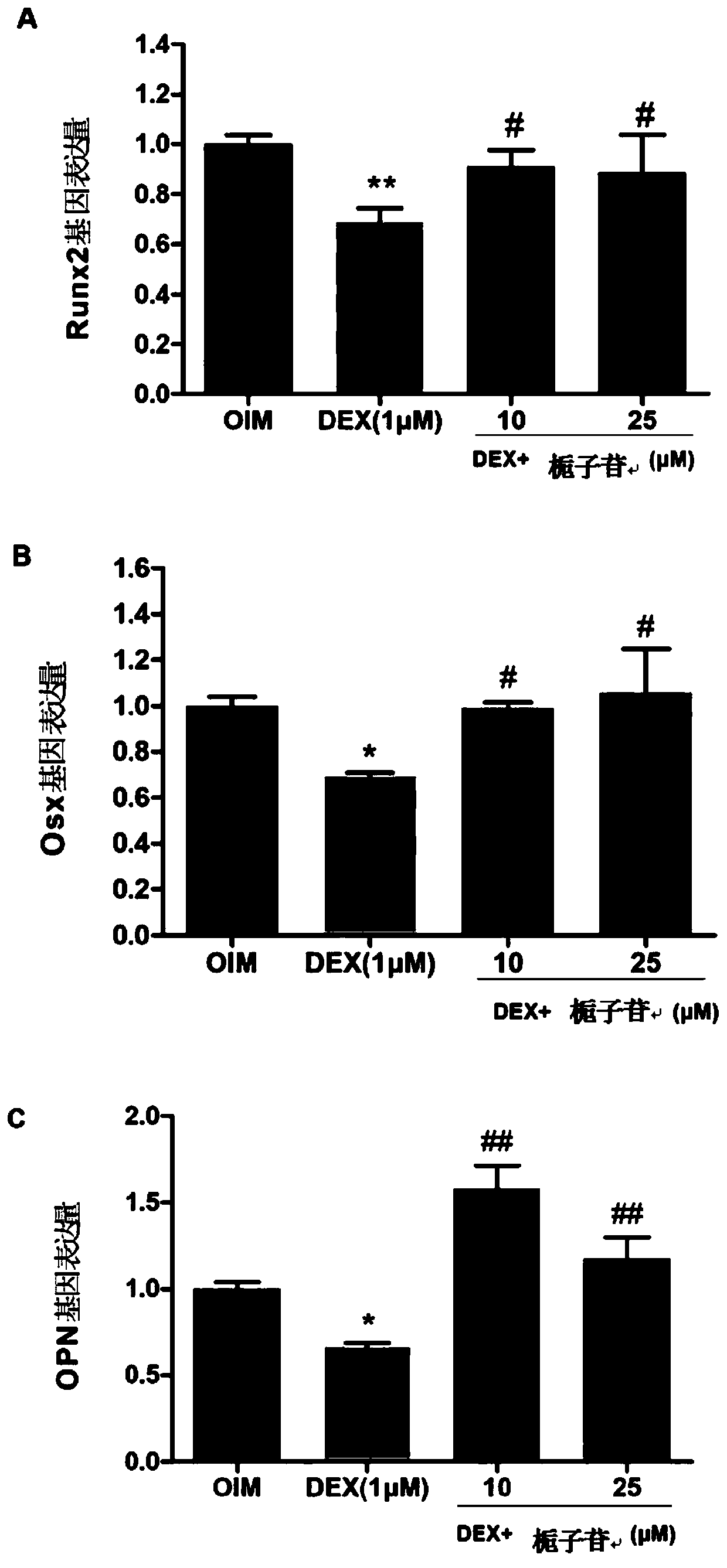
[0058] Mineralized nodules appear in the late stage of osteogenic differentiation, and are formed when hydroxyapatite crystals and amorphous calcium phosphate produced during osteoblast differentiation are secreted into the extracellular matrix and combined with osteocalcin, osteopontin, collagen, etc. Hydroxyapatite crystallization is one of the indicators of late osteogenic differentiation. Therefore, in this experiment, Alizarin red staining and calcium nodule quantification were used to study the effect of geniposide on the inhibition of MC3T3-E1 cell mineralization by dexamethasone.
[0059] Cell digestion and inoculation: take well-grown MC3T3-E1 cells, digest and count them, and use 5×10 4 Cells / well were inoculated in 24-well plates, grouped as in Example 2, transferred to a carbon dioxide incubator for culture, and after 14 days of drug treatment, the mineralization of cells was observed by ali...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com