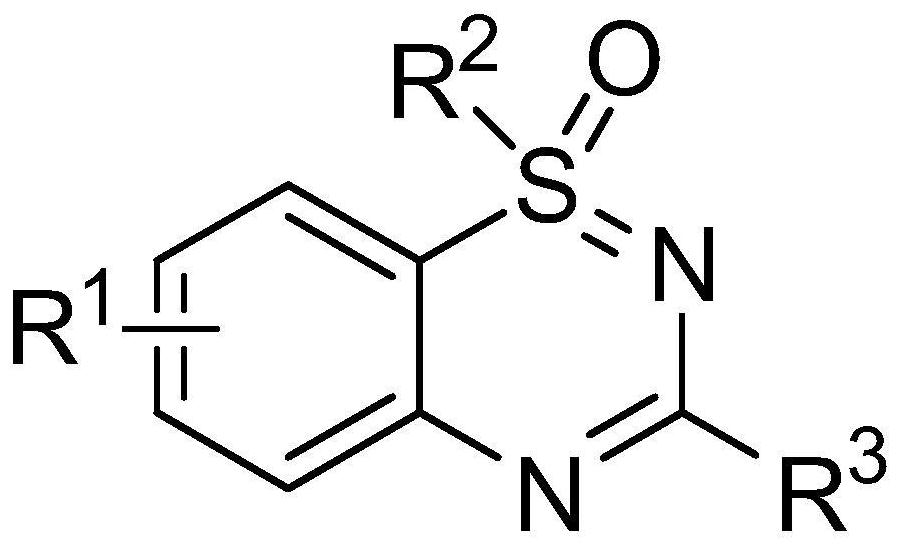
Method for synthesizing 1,2,4-benzothiadiazine series compounds in aqueous solution
A technology of benzothiadiazines and compounds, which is applied in the field of preparation of 1,2,4-benzothiadiazines compounds, can solve the problems of increased production costs and high equipment requirements, and achieves extremely easy acquisition and high product yield High, high chemoselectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
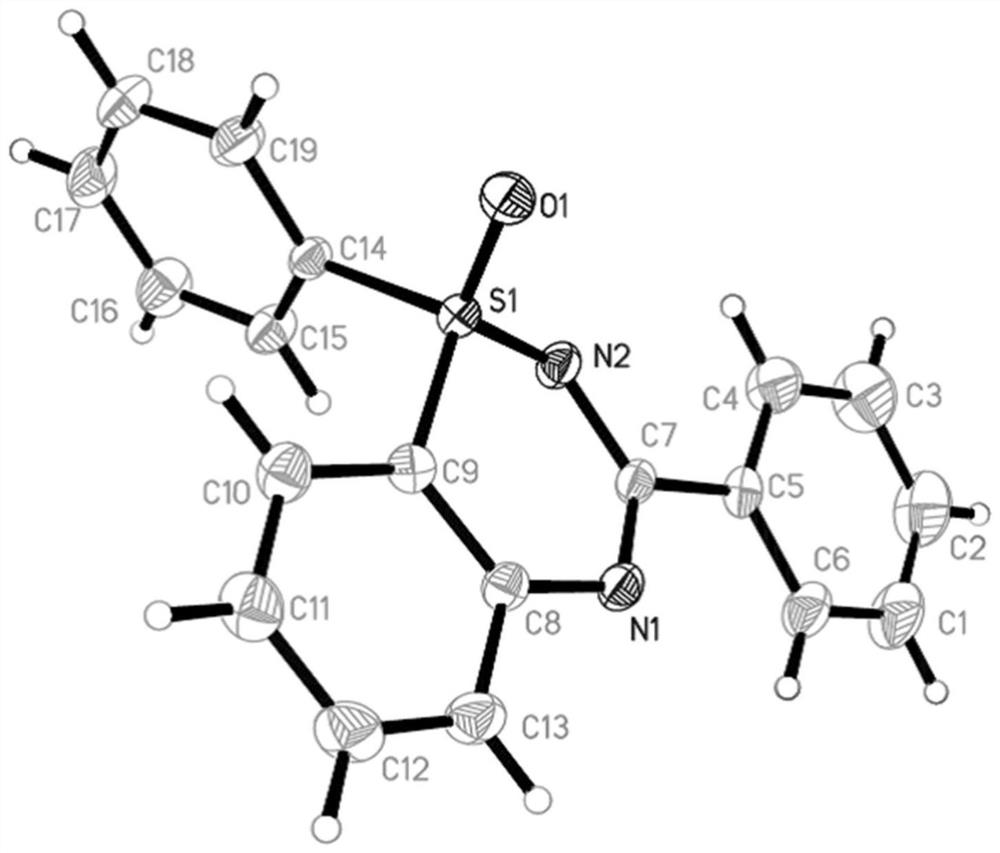
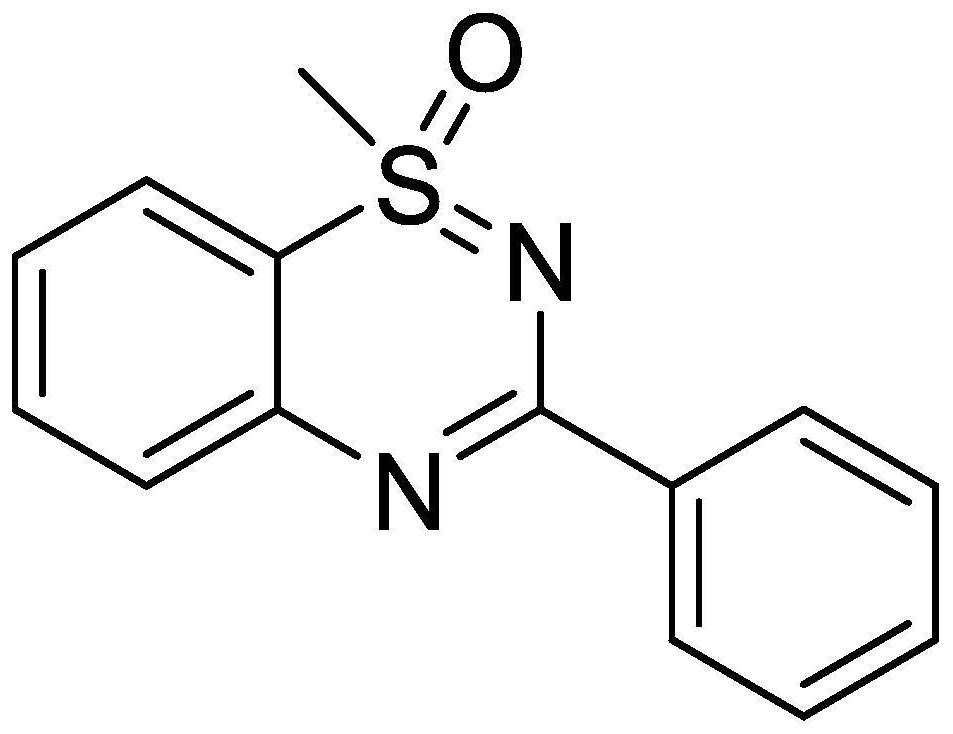
[0032] The catalyst cuprous oxide (2.1mg, 0.015mmol) and 2-bromophenylmethyliminosulfone (70.4mg, 0.3mmol) and tetrabutylammonium iodide (11.0mg, 0.03mmol) and benzaldehyde were mixed under air atmosphere (41.4 mg, 0.39 mmol) was dissolved in water (1 mL), then sodium azide (39.0 mg, 0.6 mmol) was added to seal and then placed in a 90°C environment to react for 12 hours. After the reaction was completed by TLC, it was cooled to room temperature, and water was added. (2mL), then add saturated potassium carbonate solution (2mL) and stir for 10 minutes, extract 4 times with ethyl acetate, combine the organic phases and dry with anhydrous sodium sulfate, the pure product 3a separated by flash column chromatography, the yield is 95% . The following is the NMR experimental data of product 3a:
[0033] 1 H NMR (400MHz, CDCl 3 )δ: 8.38(d,J=7.2Hz,2H),7.80(d,J=8.0Hz,1H),7.69(t,J=7.6Hz,1H),7.62(d,J=8.0Hz,1H) ,7.45(d,J=6.4Hz,3H),7.38(t,J=7.4Hz,1H),3.52(s,3H).
[0034] 13...
Embodiment 2
[0036]
[0037] The catalyst cuprous oxide (2.1g, 15mmol) and 2-bromophenylmethyliminosulfone (70.4g, 0.3mol) and tetrabutylammonium iodide (11.0g, 0.03mol) and benzaldehyde ( 41.4g, 0.39mol) was dissolved in water (1L), then sodium azide (39.0g, 0.6mol) was added to seal and then placed in a 90°C environment to react for 12 hours. After TLC detected the reaction, it was cooled to room temperature, and water ( 2L), then add saturated potassium carbonate solution (2L), stir for 10 minutes, extract 4 times with ethyl acetate, combine the organic phases and dry with anhydrous sodium sulfate, and separate the pure product 3a by flash column chromatography, the yield is 90%.
Embodiment 3
[0039]
[0040] The catalyst cuprous oxide (2.1g, 15mmol) and 2-bromophenylmethyliminosulfone (70.4g, 0.3mol) and tetrabutylammonium bromide (7.9g, 0.03mol) and benzaldehyde ( 41.4g, 0.39mol) was dissolved in water (1L), and then methanesulfonyl azide (72.6g, 0.6mol) was added to seal it and then placed in a 90°C environment to react for 12 hours. After the reaction was detected by TLC, it was cooled to room temperature and water was added. (2L), then add saturated potassium carbonate solution (2L) and stir with ethyl acetate for 4 times after stirring for 10 minutes, combine the organic phases and dry with anhydrous sodium sulfate, the pure product 3a separated by flash column chromatography, the yield is 90% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com