Cationic polymer for co-loading drugs and genes and application of cationic polymer
A technology of cationic polymers and drugs, which can be used in drug combinations, gene therapy, anti-tumor drugs, etc., can solve the problems of weak endosome escape ability, uneven particle size distribution, and poor release effect, so as to promote transmembrane transport, The preparation method is simple and the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
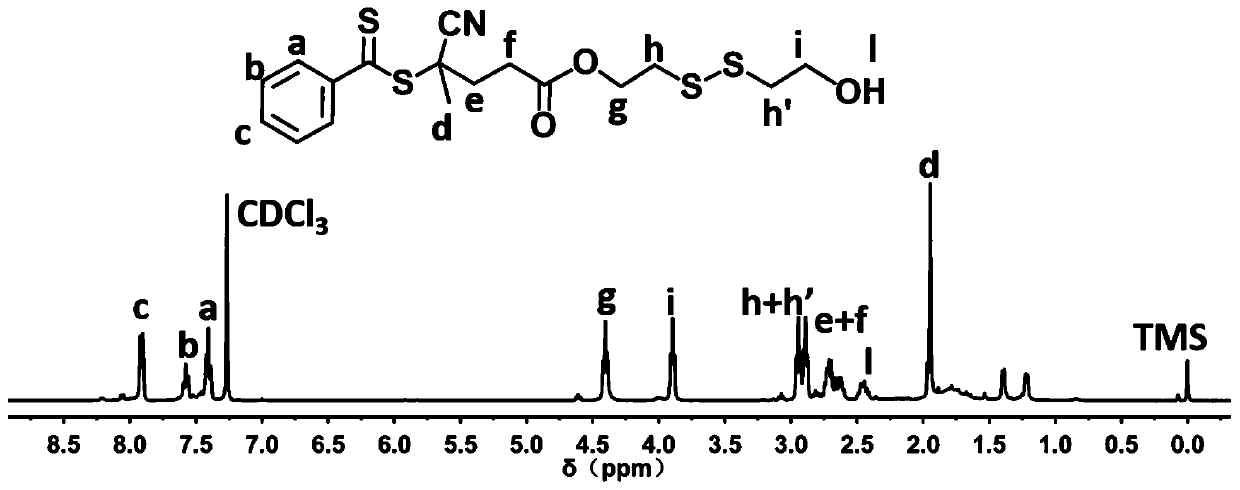
[0070] Example 1: Synthesis of 4-cyano-4-(dithiobenzoyloxy)valeric acid-dithiodiethanol ester
[0071] First, under an inert gas atmosphere, using 4-cyano-4-(dithiobenzoyloxy)valeric acid (4-CPDB) and dithiodiethanol as raw materials, the N,N ’-Diisopropylcarbodiimide (DIC) is used as water-absorbing agent and 4-dimethylaminopyridine (DMAP) as catalyst, and 4-CPDB- ss -OH double head reagent.
[0072] The specific synthesis method is as follows: During the ventilation process, dithiodiethanol (5.46 g, 35.4 mmol), dithiodiethanol (5.46 g, 35.4 mmol), and N,N ’-Diisopropylcarbodiimide (DIC, 0.88 g, 7.0 mmol), 4-dimethylaminopyridine (DMAP, 0.214 g, 1.75 mmol), and 10 mL of dried dichloromethane (CH 2 Cl 2 ); Then use a constant pressure funnel to contain 10mL CH 2 Cl 2A solution of 4-CPDB (1.0 g, 3.5 mmol) was added dropwise into a round bottom flask. After being completely sealed, the reaction bottle and constant pressure funnel were transferred to a low temperature and...
Embodiment 2
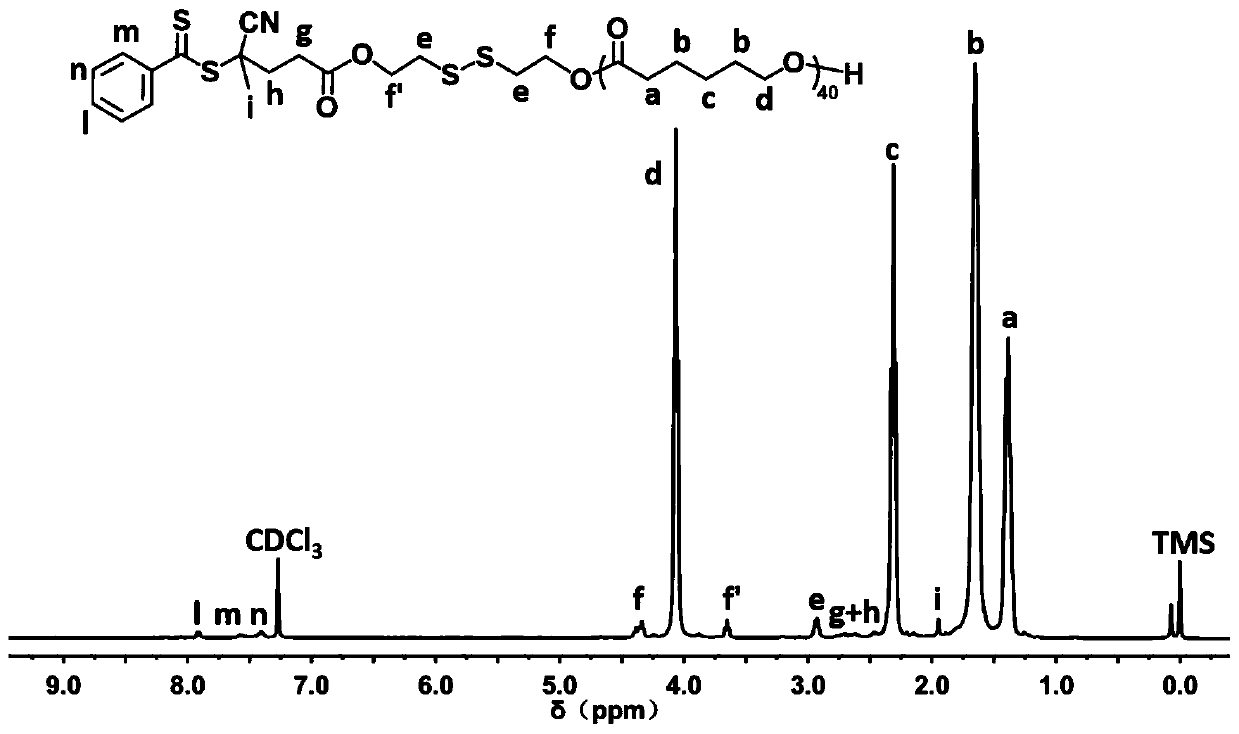
[0074] Embodiment two: polyester chain transfer agent 4-CPDB- ss -Synthesis of PCL
[0075] Place the 50 mL branched flask equipped with a stirring bar in an oven at 120 °C to dry for at least 12 h, take it out, connect the branched flask to the double-row tube, evacuate it to room temperature with an oil pump, repeat the pumping and inflation three times, and finally fill it with nitrogen. Add 4-CPDB- ss -OH (51.3 mg, 0.122 mmol), use the toluene azeotropic method to remove the residual moisture in the initiator; after the toluene distillation is completed, add ε-CL monomer (698 mg, 4.84 mmol) and stannous octoate (Sn( Oct) 2 , 24.6 mg, 0.061 mmol). After being filled with nitrogen and completely sealed, it was transferred to an oil bath at 110 °C and continued to stir for 6 h. After the reaction was completed, the reaction flask was cooled to room temperature, and a small amount of CH was added. 2 Cl 2 Dissolve the crude product, add 2-3 drops of glacial acetic acid, s...
Embodiment 3
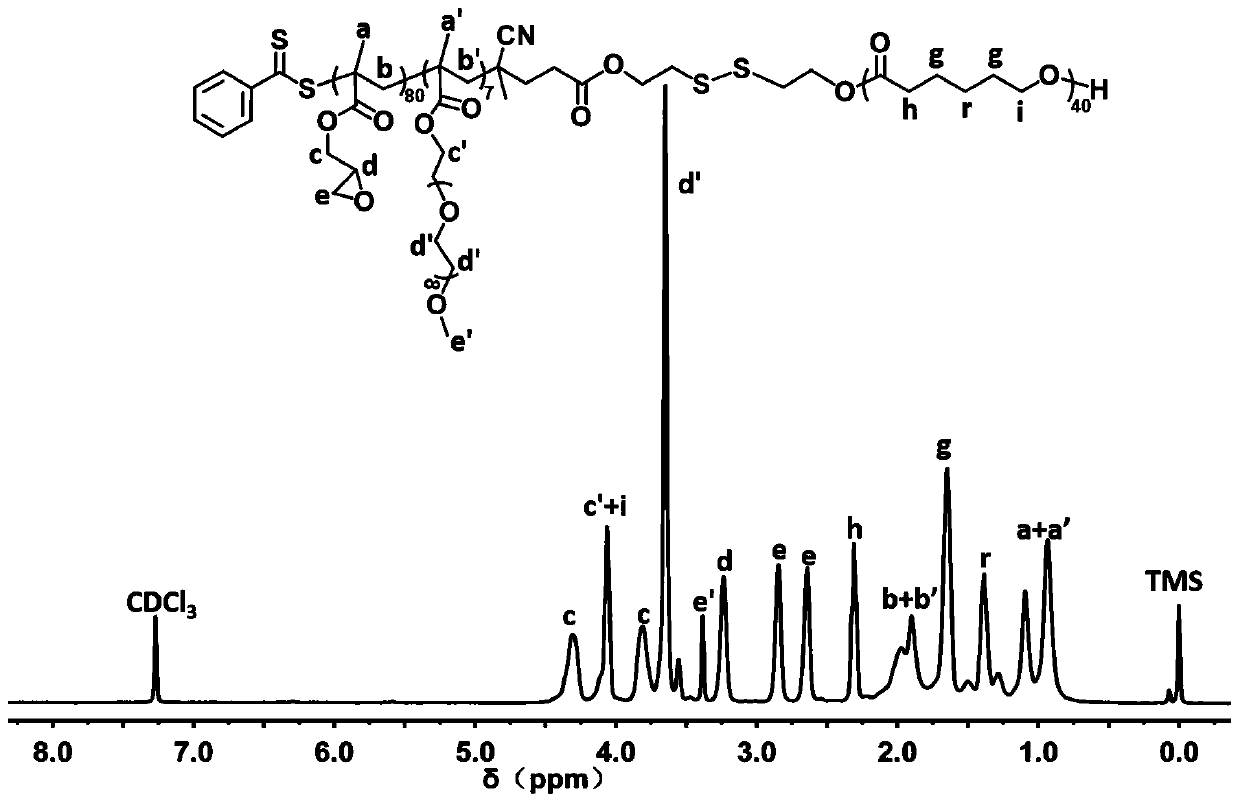
[0076] Embodiment three: polycaprolactone- ss -Poly(glycidyl methacrylate- co - Synthesis of Polyethylene Glycol Methacrylate) Copolymer
[0077] Dry the 50 mL round-bottomed flask and glass stopper with a stirring bar in an oven at 120 °C for 24 h, take it out, plug it with a glass stopper, and connect it to an oil pump through a latex tube. into high-purity nitrogen. During the aeration process, 4-CPDB- ss -PCL (100 mg, 0.020 mmol), azobisisobutyronitrile (AIBN, 3.2 mg, 0.020 mmol), polyethylene glycol methacrylate (PEGMA, 136.6 mg, 0.29 mmol), and glycidyl methacrylate (GMA, 282 mg, 1.98 mmol); under nitrogen atmosphere, add 8 mL of N,N - Dimethylformamide, evacuated, filled with nitrogen after repeating this three times. Stir until completely dissolved, then transfer to a 70 °C oil bath for 12 h. The reaction was terminated by rapid cooling. A dialysis bag with a molecular weight cut-off of 12,000-14,000 Da was used to dialyze the solution of the polymerization re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com