Covalent organic framework material containing beta-ketoenamine structure, and preparation method and application thereof
A technology of covalent organic framework and β-ketoenamine, which is applied in the field of covalent organic framework materials and their preparation, can solve the problems of difficult to meet the actual needs of electrochemical energy storage devices, low specific capacitance value of COFs materials, etc., and achieve excellent thermal performance. Stability and chemical stability, excellent rate performance and cycling stability, the effect of enriched porosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
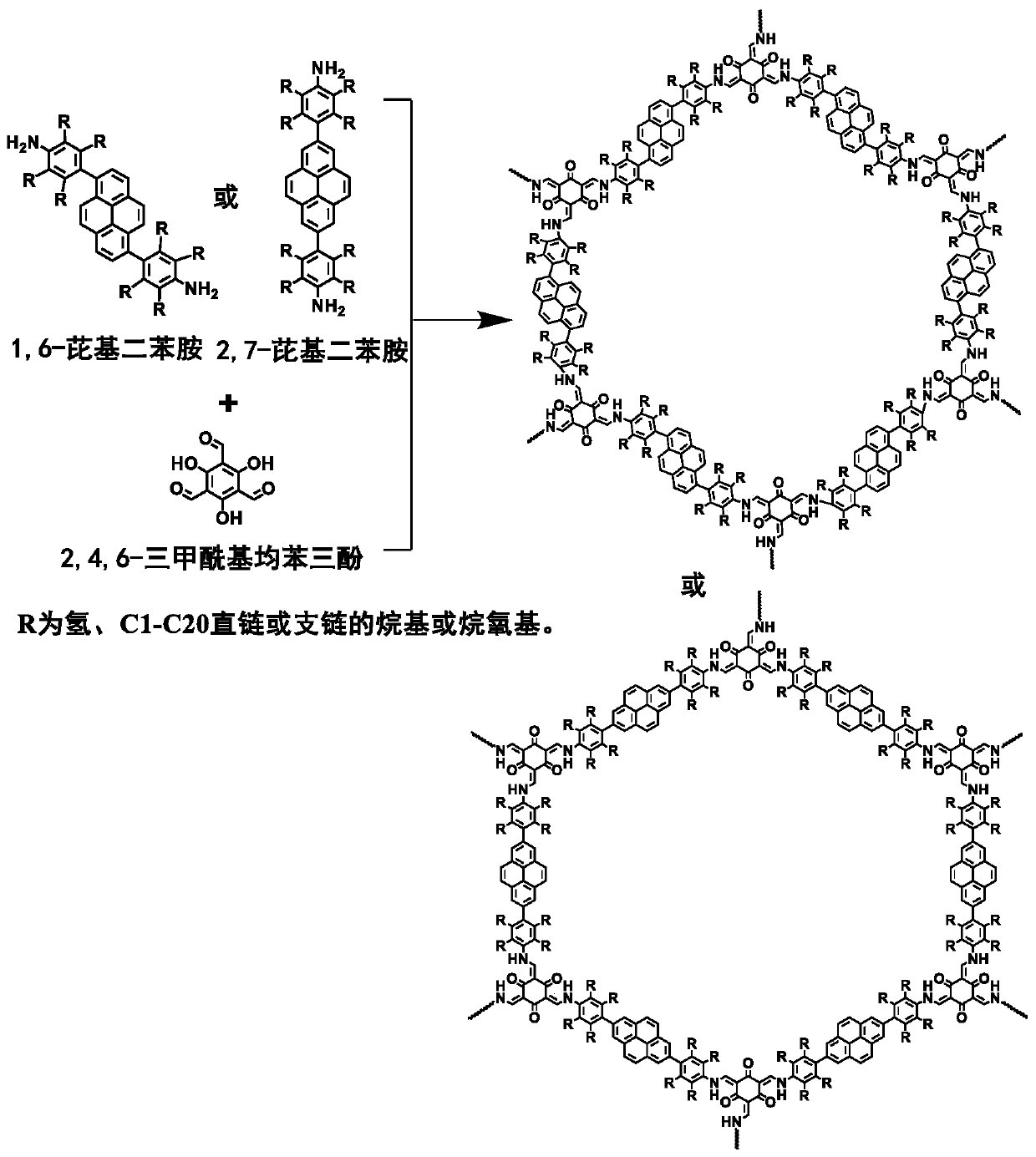
[0028] (1) Preparation of monomer 4,4'-(pyrene-1,6-disubstituted)diphenylamine:
[0029] Under nitrogen protection, 1,6-dibromopyrene (1.80g, 5.0mmol), 4-aminophenylboronic acid pinacol ester (3.28g, 15.0mmol), tetrabutylammonium bromide ( TBAB) (0.51g, 10wt%), catalyst tetrakistriphenylphosphine palladium (Pd(PPh 3 ) 4 ), adding the solvent K after bubbling deoxygenation 2 CO 3 (aq., 2M) 30mL, 1,4-dioxane 90mL, temperature-controlled reflux reaction for 72 hours. After the reaction, the crude product was purified by column chromatography with 100-200 mesh basic alumina as the stationary phase and petroleum ether / ethyl acetate (volume ratio 1:2) as the eluent to obtain 1.48 g of off-white powder with a yield of 77%.
[0030] (2) Preparation of covalent organic framework materials:
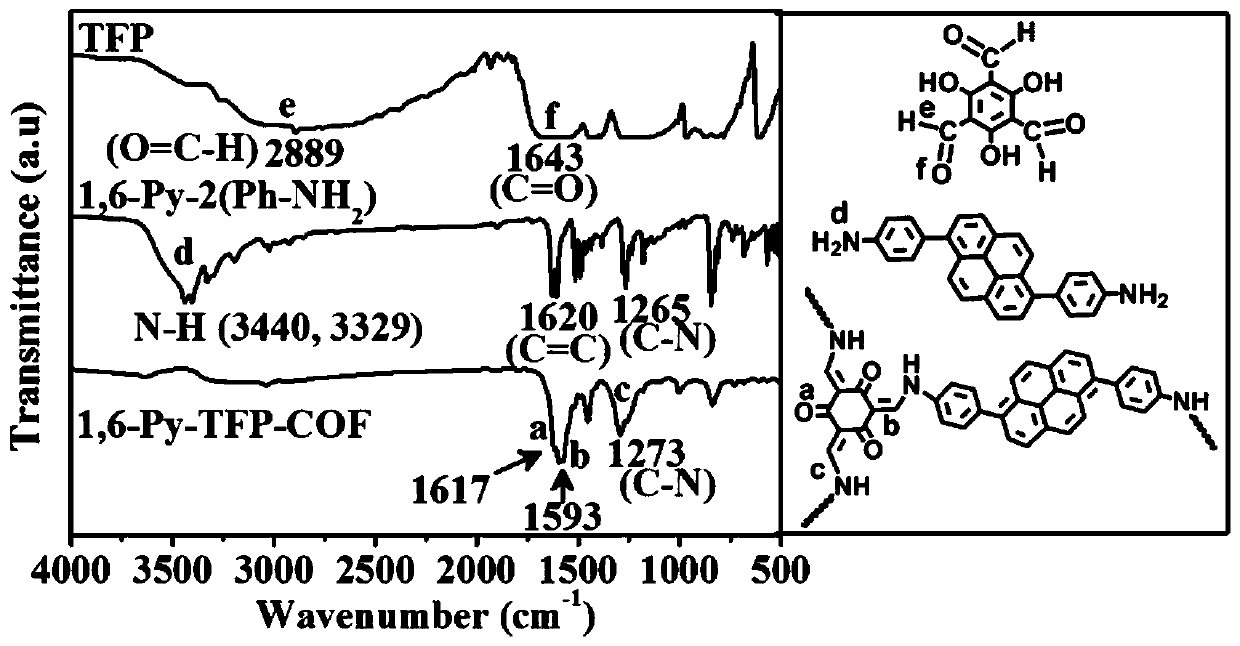
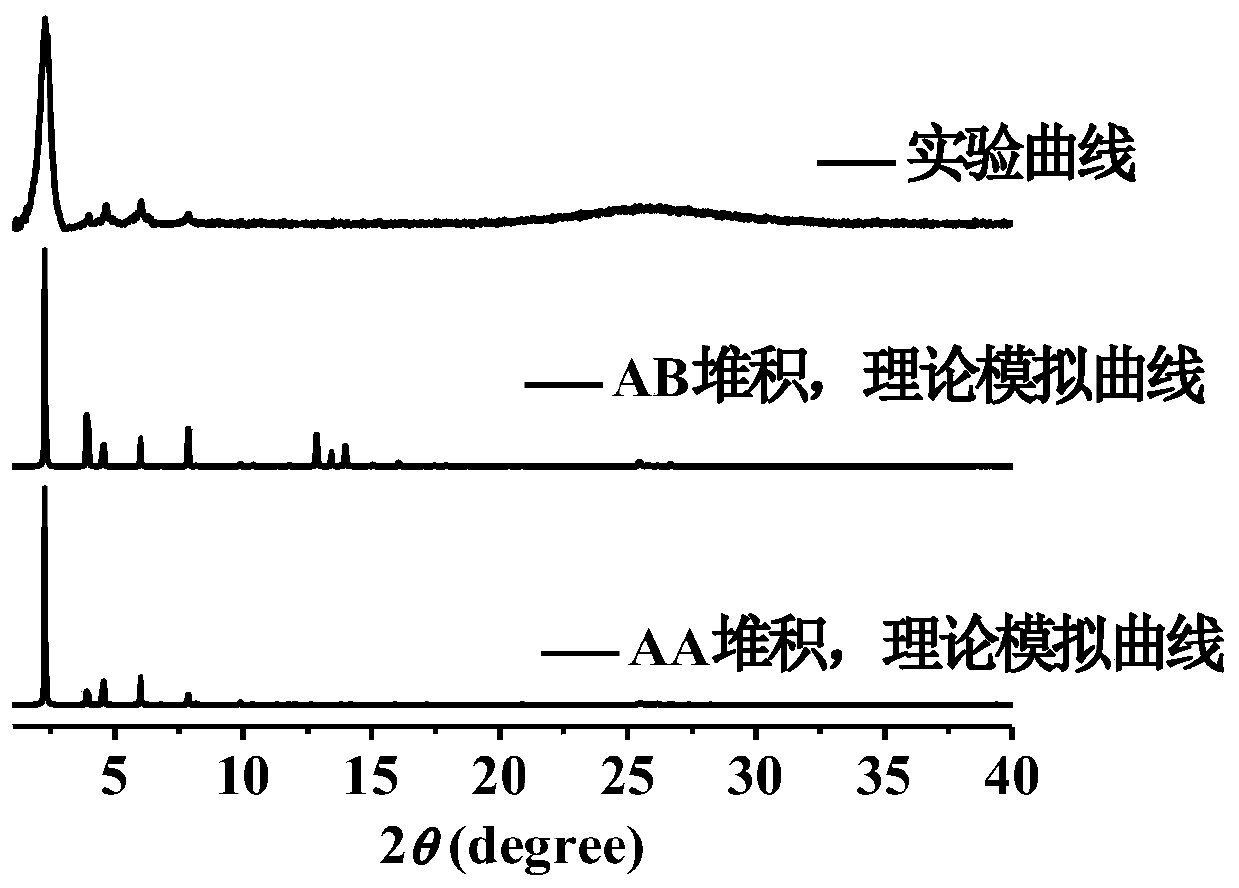
[0031] Weigh the monomer 4,4'-(pyrene-1,6-disubstituted)diphenylamine (57.6mg, 0.15mmol) synthesized in step (1), and 2,4,6-triformylpyroglucinol (TFP ) (21.0mg, 0.10mmol) into a Pyrex tube r...
Embodiment 2
[0033] The synthesis steps of the monomer 4,4'-(pyrene-1,6-disubstituted)diphenylamine are the same as the step (1) in Example 1, and the 4,4'-(pyrene-1,6-disubstituted) Put 57.6mg of diphenylamine and 21.0mg of 2,4,6-triformylpyroglucinol (TFP) into a high temperature and high pressure resistant Pyrex tube, add 2mL of o-dichlorobenzene and 1mL of n-butanol, and sonicate for 2min to form a uniform dispersion Then add 0.3mL of 6M acetic acid aqueous solution as an acidic catalyst, seal the Pyrex tube and put it into a liquid nitrogen bath for quick freezing, then evacuate until the pressure inside the tube reaches 0.15mmHg, and then thaw to remove the air in the solvent; Quick freezing-vacuumizing-thawing operation cycles more than 3 times; finally, the reaction tube was heated to 120°C and left to react for 5 days. After the reaction, the solid obtained by suction filtration was extracted by Soxhlet with anhydrous tetrahydrofuran for more than 24 hours, and finally the solid w...
Embodiment 3
[0035] The synthesis steps of monomer 4,4'-(pyrene-1,6-disubstituted)diphenylamine are the same as step (1) in Example 1, and 4,4'-(pyrene-1,6-disubstituted)diphenylamine is weighed Put 57.6mg of aniline and 21.0mg of 2,4,6-triformylpyroglucinol (TFP) into a Pyrex tube resistant to high temperature and high pressure, add 1mL of o-dichlorobenzene and 2mL of n-butanol, and sonicate for 2min to form a uniform dispersion , then add 0.3mL of 6M acetic acid aqueous solution as an acidic catalyst, seal the Pyrex tube and put it into a liquid nitrogen bath for quick freezing, then evacuate until the pressure in the tube reaches 0.15mmHg, and then thaw to remove the air in the solvent; quick freeze the liquid nitrogen bath - Vacuum pumping - thawing operation cycle for more than 3 times; finally, heat the reaction tube to 120°C and let it stand for reaction for 5 days. After the reaction, the solid obtained by suction filtration was extracted by Soxhlet with anhydrous tetrahydrofuran f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com