Terminal alkyne-containing benzophenone derivative and preparation method and application thereof
A technology of dihydroxybenzophenone and benzophenone, which is applied in the field of benzophenone derivatives and its preparation, can solve problems such as limited applications, achieve enhanced light stability, good compatibility, and reduce mobility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
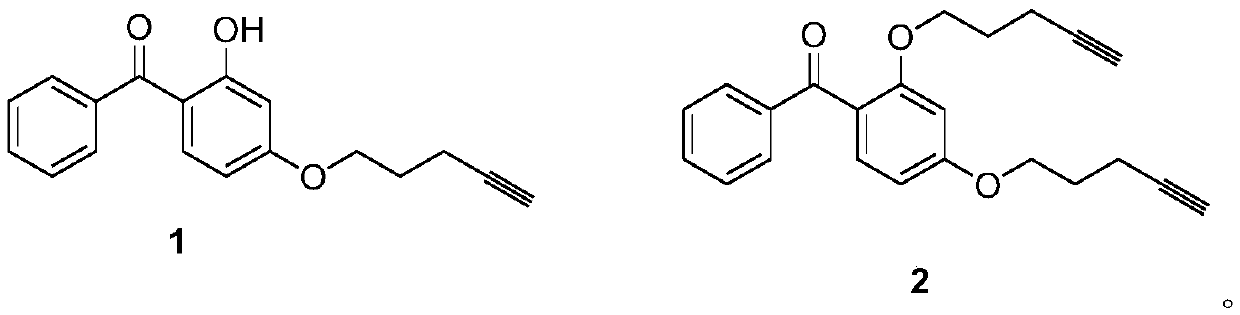
[0020] Add 0.214g (1mmol) of 2,4-dihydroxybenzophenone and 0.207g (1.5mmol) of potassium carbonate to the container, dissolve it with 3ml of N,N-dimethylformamide, and then add 0.205g ( 2 mmol) of 5-chloro-1-pentyne, stirred at 85°C for 12 hours. After the reaction, add water to the reaction solution and extract with ethyl acetate, collect the organic phase, wash with water, dry over anhydrous sodium sulfate, and remove the organic solvent by rotary evaporation; and use petroleum ether and ethyl acetate (volume ratio 4:1) as The eluent is separated by column chromatography to obtain the benzophenone derivative 1 containing the terminal alkyne and the benzophenone derivative 2 containing the terminal alkyne.
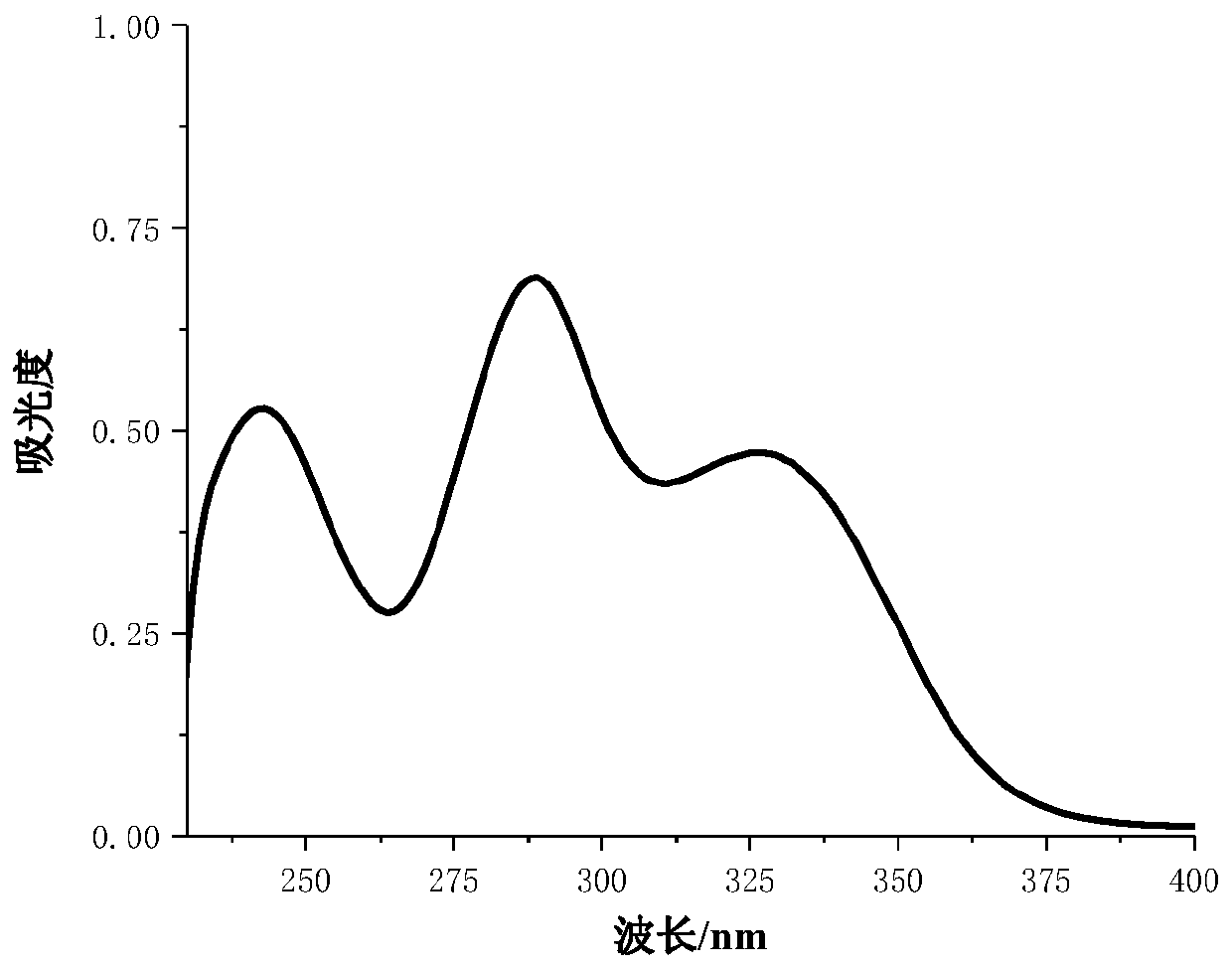
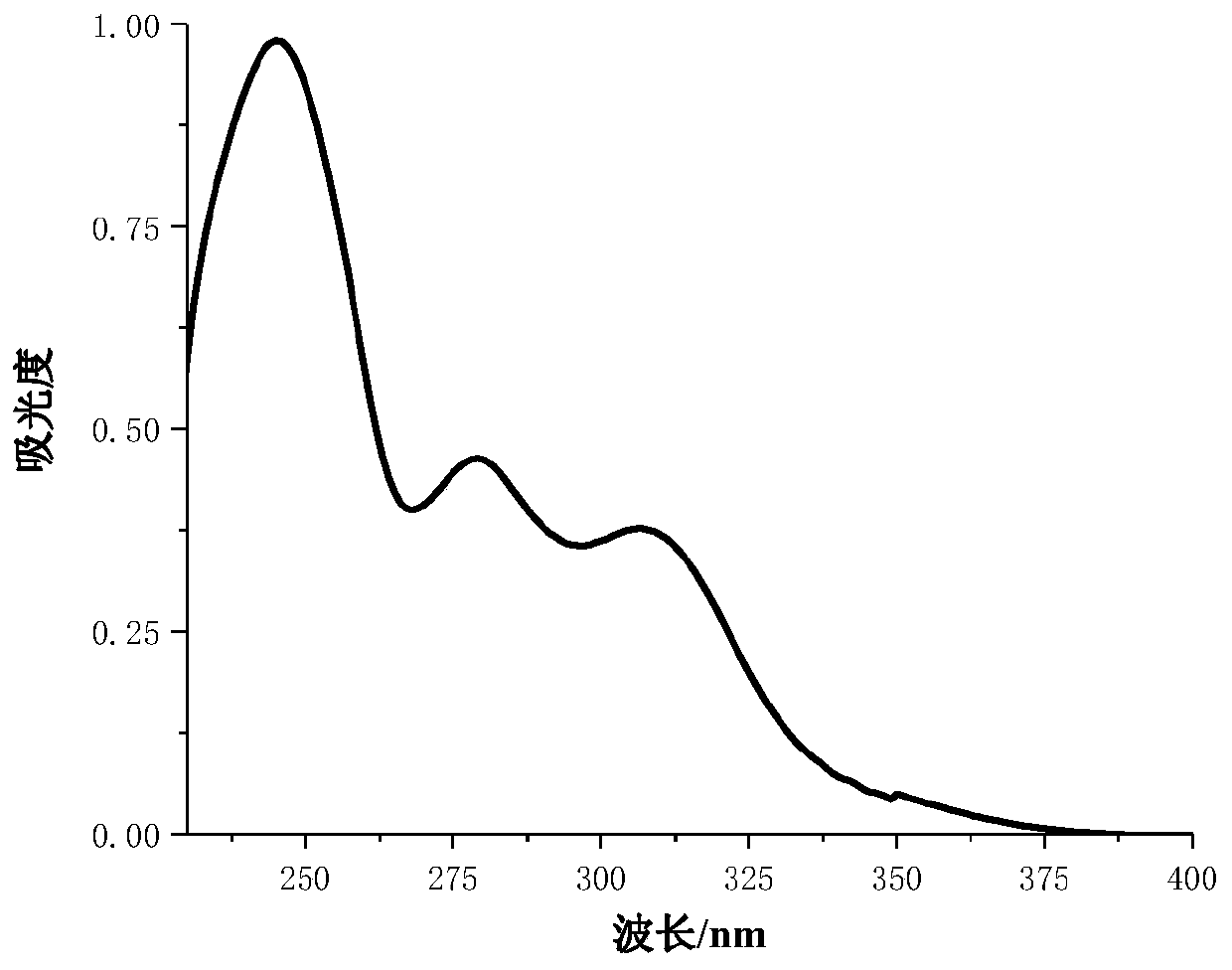
[0021] 2-Hydroxy-4-(pent-4-yne-1-oxyl)benzophenone (derivative 1): white solid, yield: 45%, m.p.25-27°C. 1 H NMR (600MHz, CDCl 3 )δ12.72–12.69(m,1H),7.68–7.63(m,2H),7.61–7.56(m,1H),7.54–7.49(m,3H),6.55(d,J=2.5Hz,1H) ,6.43(dt,J=10.2,5.1Hz,1H),4.19–4.14(m,2H),2.08–2.02(m...
Embodiment 2
[0025] Embodiment 2: Derivative 1 is used in the performance evaluation of ultraviolet absorber
[0026] Experimental recipe:
[0027]
[0028] Working conditions:
[0029] Add 50g of epoxy propylene carboxylic acid resin, 44g of 1,6-hexanediol diacrylate, 3g of photoinitiator (1173), 2g of triethanolamine, 2-hydroxy-4-(pentane) in the glass container equipped with stirrer - 1g of 4-alkyne-1-oxyl)benzophenone, stirred to make it dispersed uniformly and transparently, and stood still for 5-10 minutes to obtain a transparent photocurable coating. The paint is divided into three parts, one part is put into a transparent glass bottle and covered for daily indoor storage; the other part is put into a transparent glass bottle and covered with a cover for indoor dark storage; one part is coated on a glass plate with a brush, and the film The thickness is 75 μm, and then cured by a UV curing instrument at a speed of 5 meters per minute (about 5 seconds of light time).
Embodiment 3
[0054] Embodiment 3: Applied performance evaluation of derivative 2
[0055] Experimental formula:
[0056]
[0057] working conditions
[0058] Under the condition of avoiding light, add 0.03g of 2,4-di(pent-4-yn-1-oxyl)benzophenone and 0.5g of modified epoxy acrylate (UV1005-65) into the glass container, 1, 0.45g of 6-hexanediol diacrylate (HDDA), 0.02g of triethanolamine, and stir evenly until the coating liquid becomes transparent. The mixture was coated on a glass plate with an applicator to form a film with a film thickness of 75um. It was irradiated and cured in a medium-pressure mercury lamp, and the power of the mercury lamp was 400W.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com