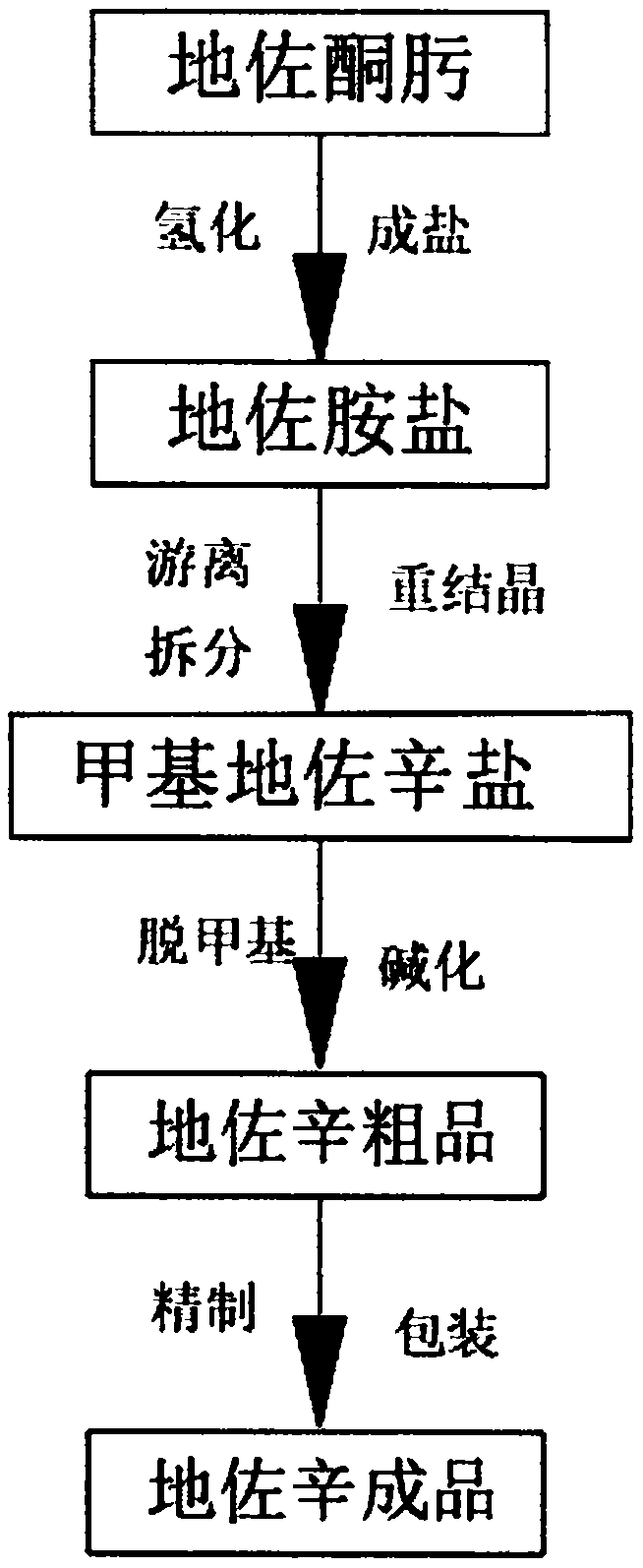
Dezocine production process
A technical process, the technology of dezocine, applied in the field of medicinal chemistry, can solve the problems of low reaction safety, harsh reaction conditions, long synthesis cycle, etc., and achieve the effect of improving the quality of finished products, shortening the synthesis cycle, and increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
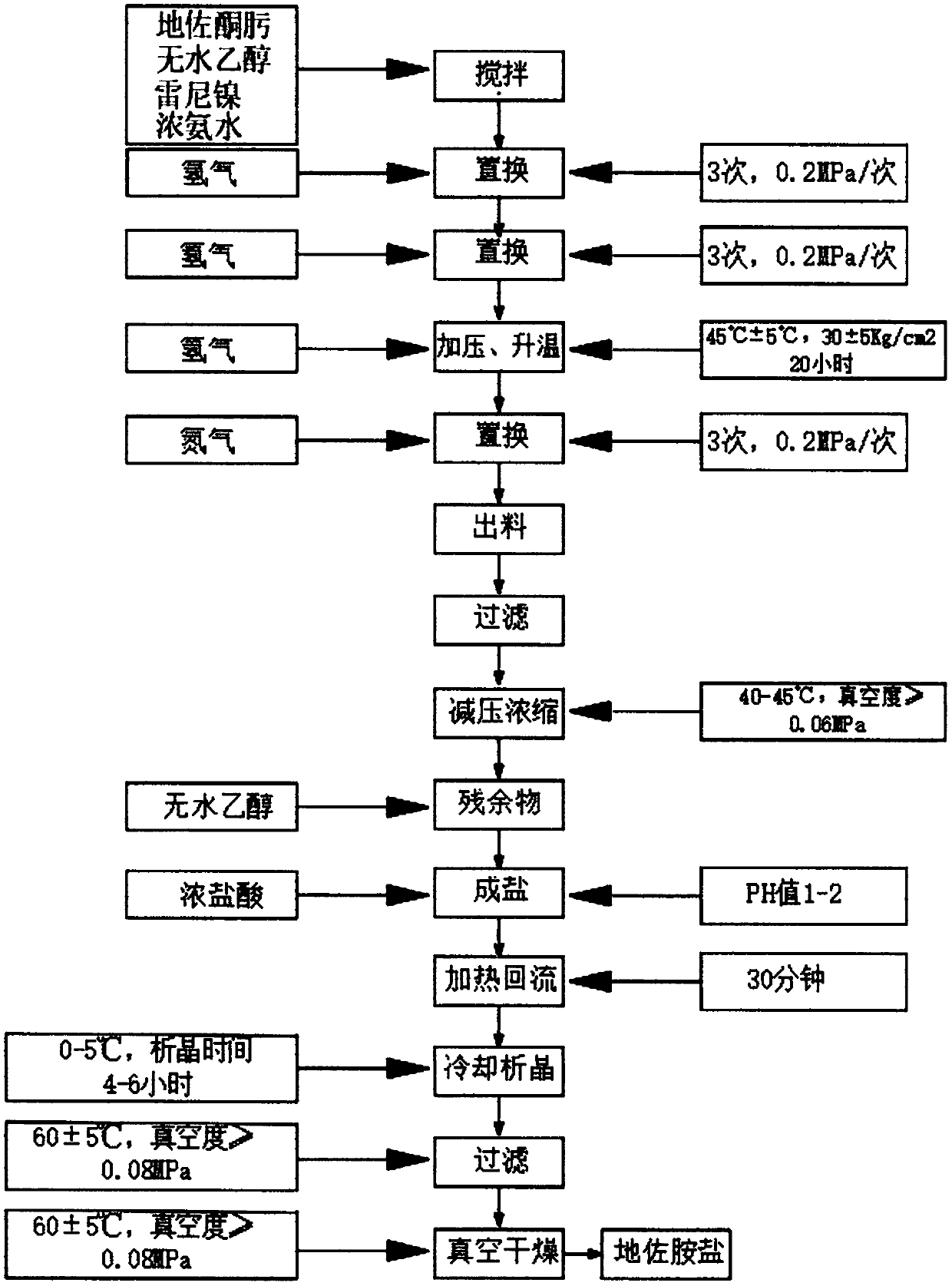
[0048] Such as figure 2 As shown, the embodiment of the present invention provides the synthesis process of diazolamide salt comprising the following steps:
[0049] 1) Ruan's nickel process: First, add 78kg of purified water to the SF-100L reaction kettle with an opening, start stirring, slowly add 32.8kg of sodium hydroxide, stir and dissolve, then turn on the cooling device and cool down to 50°C , turn off the cooling device, add 7.87kg of 50% aluminum-nickel alloy in batches under stirring, then, after adding, stir for 10 minutes, turn on the heating device, raise the temperature to 97°C, keep stirring for 30 minutes, turn off the heating device, then turn on the cooling device, and cool down to 40°C, stop stirring, let it stand for 15 minutes, absorb the upper alkali water layer with vacuum, wash with purified water 3 times, 40kg each time, until the pH is 7.5, and finally put it in a stainless steel bucket, and finally, wash with absolute ethanol 2 times, 4L each time,...
Embodiment 2
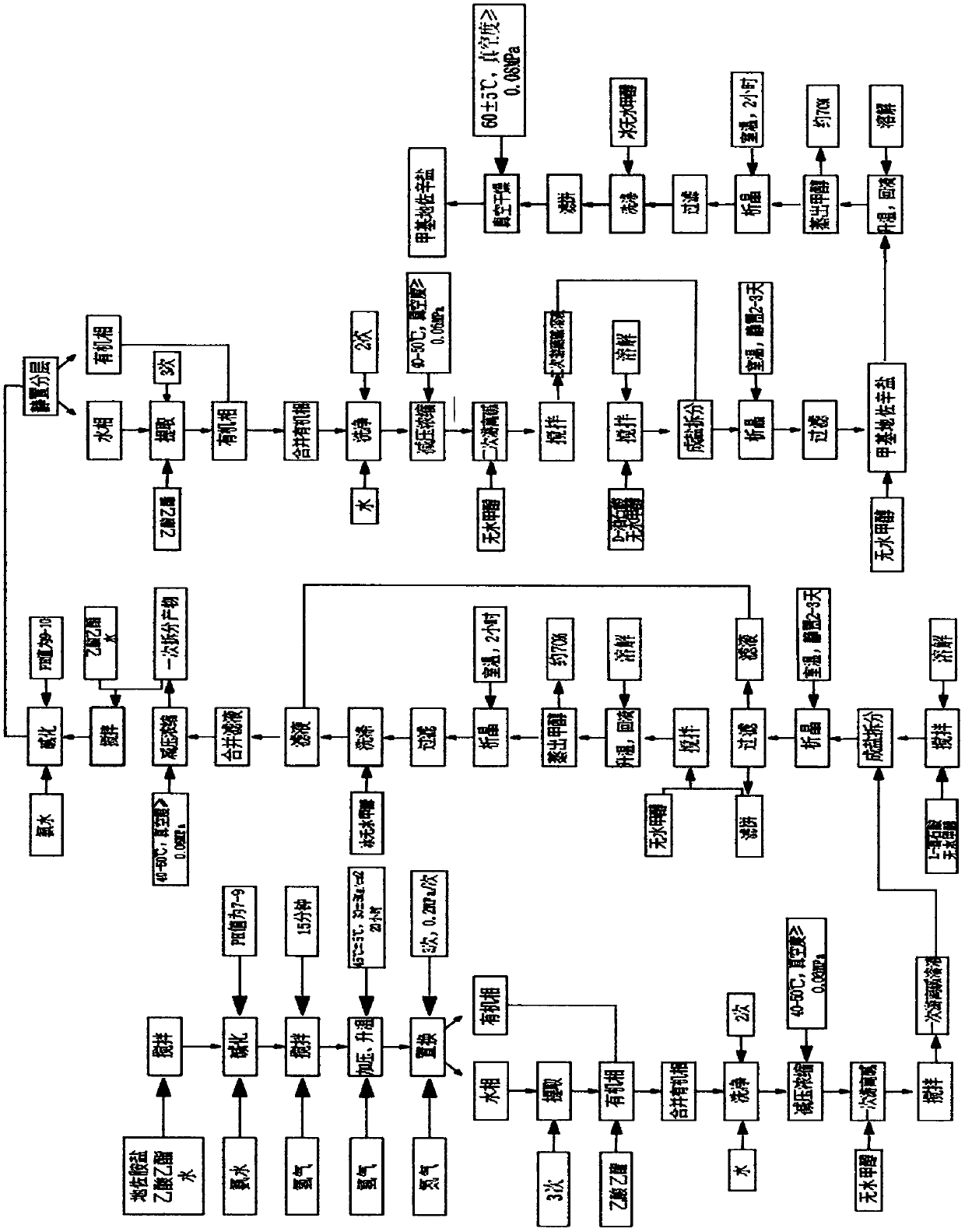
[0053] Such as image 3 As shown, the embodiment of the present invention provides a synthesis process of methyl dezocine salt comprising the following steps:
[0054] 1) Primary dissociation: First, add 5.76kg of diazolamide salt, 28kg of purified water and 24kg of ethyl acetate into the SF-100L reactor, start stirring, add concentrated ammonia water dropwise until the pH value is 9.5, and stir for 15 minutes , then, turn off the stirring, let stand to separate the layers, separate the organic layer, extract the aqueous layer with ethyl acetate 3 times, 7.5L each time, combine the organic phases, and finally, wash the organic phase twice with purified water, 7.5L each time, The organic phase was concentrated under reduced pressure, and the solvent was evaporated to obtain an oily substance, that is, a primary free base;
[0055] 2) One-time splitting: First, add 2.91kg of L-tartaric acid and 37.4kg of anhydrous methanol into the SF-100L reactor, start stirring, after stirrin...
Embodiment 3
[0060] Such as Figure 4 As shown, the synthesis process of crude dezocine provided by the embodiment of the present invention includes the following steps:
[0061] 1) Demethylation process: In the SF-100L reactor, add 1.82kg of methyl dezocine salt and 16L of 48% hydrobromic acid, start stirring, pass in nitrogen protection, turn on the heating device, and wait until the reflux is stable. Reflux reaction for 2 hours, turn off the heating device, turn on the cooling device, then, when the temperature drops to about 80°C, turn off the cooling device, turn on the heating device and the water circulation vacuum system, evaporate the solvent to obtain a solid, filter it while it is hot, and use 400ml of purified water After washing, the filtrate was cooled to room temperature, placed in a freezer, cooled and crystallized for more than 4 hours, and finally, filtered to obtain dezocine hydrobromide solid;
[0062] 2) Alkalization process: first, in the SF-100L reactor, add dezocin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com