Multi-core co-shell compound drug-carrying microsphere, and preparation method and application thereof
A drug-loaded microsphere and multi-core technology, which is applied in the fields of application, pharmaceutical formulation, and drug combination, can solve the problems of drug burst release and drug loading rate reduction, and achieve improved utilization, good biocompatibility, and reduced burst release effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The second aspect of the present invention provides a method for preparing the above-mentioned multi-core co-shell composite drug-loaded microspheres, the steps comprising:
[0043] S1. Heat and dissolve the oil-soluble PHBV in an organic solvent, and then add FNS sonication to dissolve it in the organic solvent to obtain a drug-loaded PHBV solution;
[0044] S2. Add the drug-loaded PHBV solution obtained in step S1 into the polyvinyl alcohol solution, and stir to form an O / W emulsion;
[0045] S3. Add the O / W emulsion obtained in step S2 dropwise into the polyvinyl alcohol solution, stir until the organic solvent volatilizes to obtain the drug-loaded microspheres, and freeze-dry them after washing;
[0046] S4. Add the FNS / PHBV microspheres obtained in step S3 to the PVA / CS mixed solution containing Tween80, stir and sonicate to form a S / W mixed phase;
[0047] S5. Add the S / W mixed phase obtained in step S4 into the pre-emulsified oil phase, stir to form a S / W / O emul...
Embodiment 1
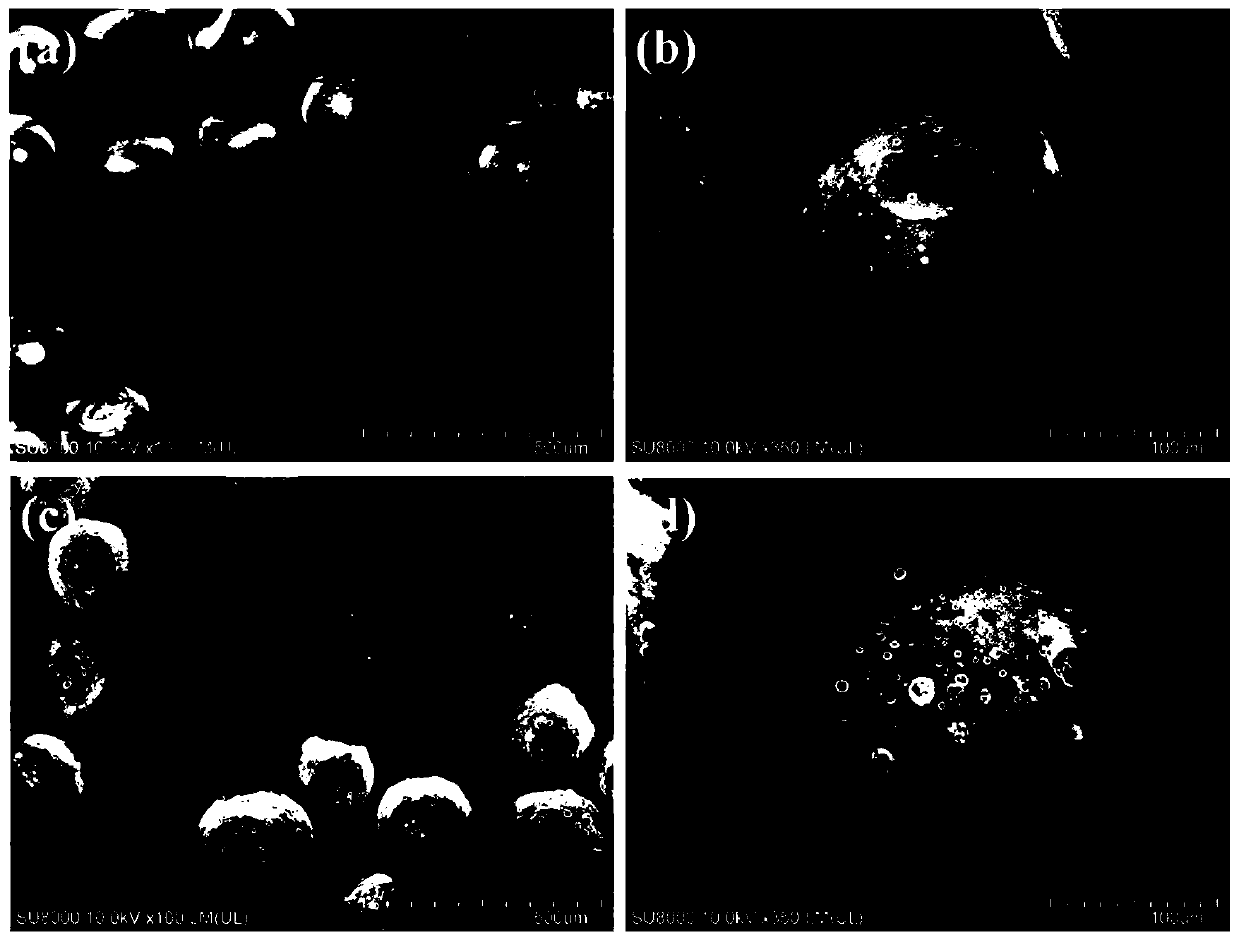
[0066] Weigh 160mg PHBV (PHV is 12mol%) in the mixed organic solvent that is equipped with 4mL dichloromethane / ethyl acetate (dichloromethane / ethyl acetate=70:30, v / v), the water bath is heated to 40 ℃ fully Dissolve, add 80mg FNS immediately after cooling, and dissolve the drug by ultrasonication for 8min. Add the above FNS / PHBV solution into a glass sample bottle containing 16mL 1wt% PVA1788, use a high-speed homogenizer to homogeneously stir at 8000rpm for 2min to form an O / W emulsion, and then add it to a three-necked flask containing 160mLPVA1788 after standing for 5min , stirred mechanically at 480 rpm for 5 h at room temperature, collected the drug-loaded microspheres by centrifugation, washed three times with deionized water, and stored after freeze-drying. The morphology and structure of the drug-loaded microspheres were observed and analyzed by field emission scanning electron microscopy (FESEM). attached by figure 1 It can be seen that the drug-loaded microspheres...
Embodiment 2
[0069] Weigh 0.8g of PVA124 and add 5.85mL of deionized water in a water bath to heat to dissolve, meanwhile weigh 0.04210g of CS (molecular weight is 110kDa) and dissolve in 5mL of 1wt% acetic acid solution. The two were mixed under magnetic stirring, and then a certain volume of Tween80 was added into the PVA / CS mixed solution to make the final concentration 0.5 wt%, and the magnetic stirring was continued for 20 min. Disperse the FNS / PHBV microspheres prepared in Example 1 in 3.5mL of 1wt% polyvinyl alcohol 1788 solution, then add the PVA / CS mixed solution dropwise under magnetic stirring, and then perform ultrasonication for 30min after magnetic stirring for 20min to form a uniform S / W mixed phase. The S / W mixed phase was slowly added dropwise to 72 mL of n-heptane with a mass fraction of 2.43% Span80 (pre-emulsified for 30 min), magnetically stirred at 350 rpm at room temperature for 30 min to form a S / W / O emulsion, and 25 wt. % glutaraldehyde solution (ether: 4.15 mL, g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com