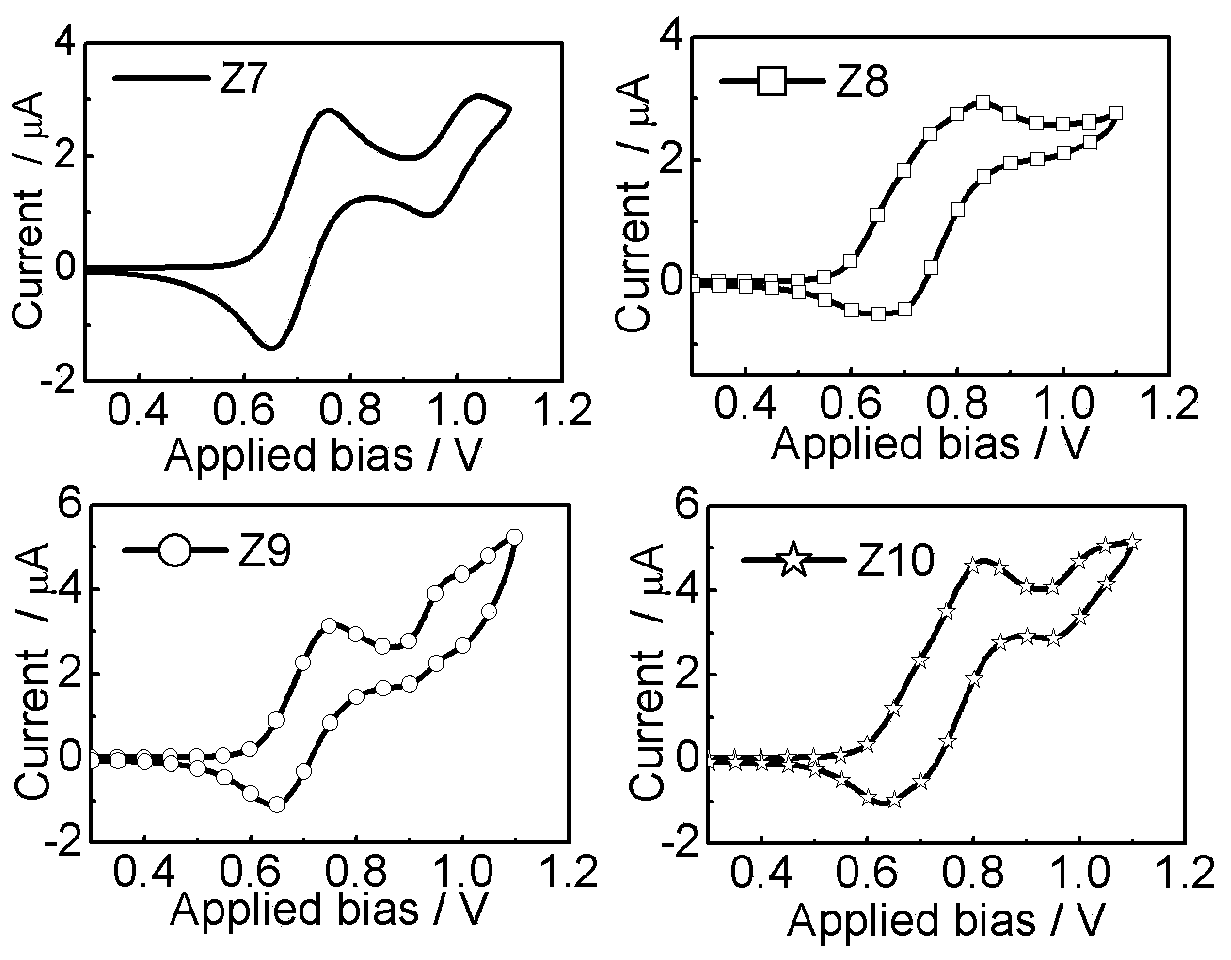
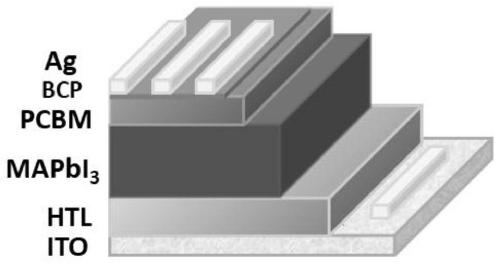
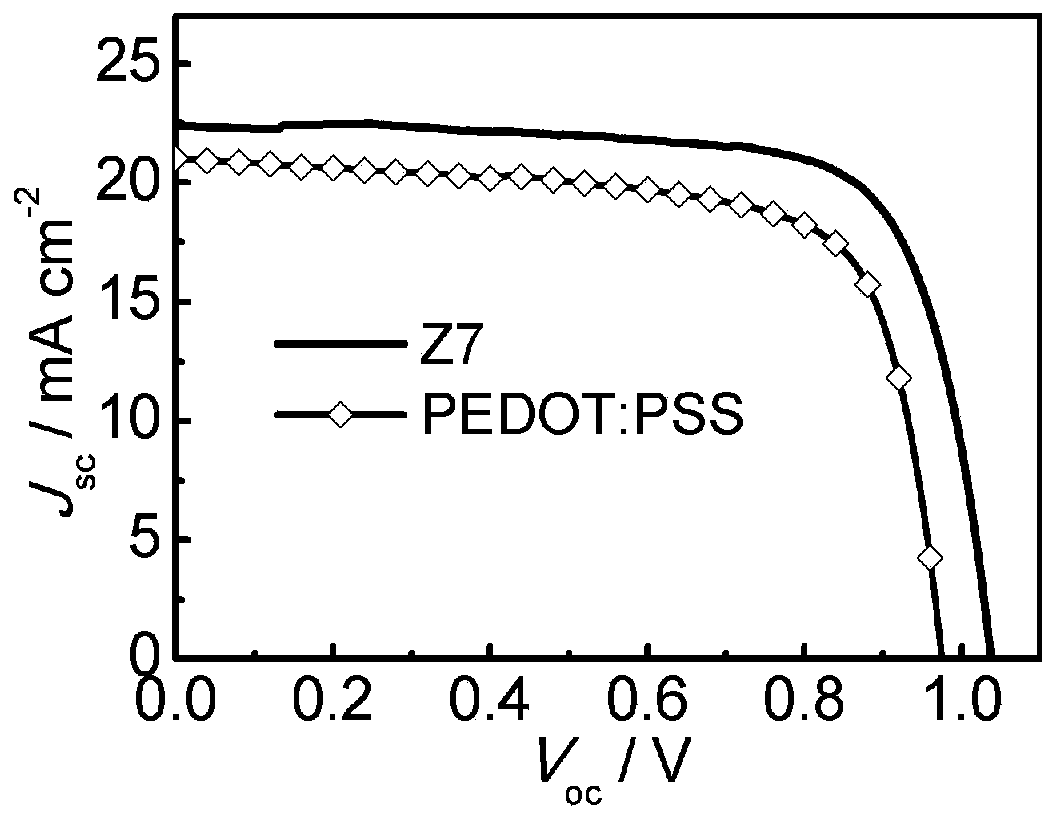
Triphenylamine type organic hole transport material with 1,1'-bi-2-naphthylamine as parent nucleus and synthesis and application of triphenylamine type organic hole transport material
A hole transport material, binaphthylamine technology, applied in the field of synthesis of triphenylamine organic small molecules, to achieve the effect of reducing the recombination of electrons, high yield and good shape stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthetic route of triphenylamine organic small molecule 3 with binaphthylamine as the core:
[0034]
[0035] In a 100mL two-necked flask, add 0.417g 6,6'-dibromo-[1,1'-binaphthyl]-2,2'-diamine 1, 1.059g triphenylamine borate 2, 0.054g tetra (Triphenylphosphine)palladium, 15ml tetrahydrofuran (THF), stirred for 5 minutes under nitrogen protection. Subsequently, the prepared sodium carbonate solution (8 mL of water + 0.400 g of anhydrous sodium carbonate powder) was added to the mixture. The resulting solution was stirred at reflux for 20 hours under nitrogen protection. After cooling, the solvent was distilled off under reduced pressure. The crude product was extracted, separated and dried, and finally separated by column chromatography (eluent: petroleum ether / ethyl acetate=2 / 1) to obtain 0.267 mg of 6,6'-bis(4-(bis(4- Methoxyphenyl)amino)benzene)-[1,1'-binaphthyl]-2,2'-diamine 3, yield 64%. 1 H NMR (400MHz, d 6 -DMSO)δ(ppm): 7.97(s, 2H), 7.82(d, J=9.16Hz, 2H)...
Embodiment 2
[0037] Synthetic route of triphenylamine organic small molecule 5 with binaphthylamine as the core:
[0038]
[0039] In a 100mL two-necked bottle, add 0.540g 6,6'-dibromo-N 2 ,N 2 ,N 2’ ,N 2’ -Tetramethyl-[1,1'-binaphthyl]-2,2'-diamine 4, 1.216g triphenylamine borate 2, 0.054g tetrakis(triphenylphosphine)palladium, 15mL tetrahydrofuran (THF) , and stirred for 5 minutes under nitrogen protection. Subsequently, the prepared sodium carbonate solution (8 mL of water + 0.400 g of anhydrous sodium carbonate powder) was added to the mixture. The resulting solution was stirred at reflux for 20 hours under nitrogen protection. After cooling, the solvent was distilled off under reduced pressure. The crude product was extracted, separated and dried, and finally separated by column chromatography (eluent: petroleum ether / ethyl acetate=5 / 1) to obtain 0.270 mg of 6,6'-bis(4-(bis(4- Methoxyphenyl)amino)benzene)-N 2 ,N 2 ,N 2’ ,N 2’ -Tetramethyl-[1,1'-binaphthyl]-2,2'-diamine 5, ...
Embodiment 3
[0041] Synthesis of triphenylamine organic small molecule 6 with binaphthylamine as the core:
[0042]
[0043] In a 100mL two-necked bottle, add 0.300g 6,6'-bis(4-(bis(4-methoxyphenyl)amino)benzene)-[1,1'-binaphthyl]-2,2'- Diamine 3, 0.237g 4-iodoanisole, 0.00156g tris(dibenzylideneacetone)dipalladium, 0.00241g 2-cyclohexylphosphine-2',4',6'-triisopropylbiphenyl, 0.115g potassium tert-butoxide and 10mL tert-butanol were heated and stirred at 95°C for 30 hours under the protection of nitrogen. After cooling, the solvent was distilled off under reduced pressure. The crude product was extracted, separated and dried, and finally separated by column chromatography (eluent: petroleum ether / ethyl acetate=1 / 1) to obtain 0.219g 6,6'-bis(4-(bis(4- Methoxyphenyl)amino)benzene)-N 2 ,N 2’ -Bis(4-methoxyphenyl)-[1,1'-binaphthyl]-2,2'-diamine 6, yield 73%. 1 H NMR (400MHz, d 6 -DMSO) δ (ppm): 8.05 (s, 2H), 7.91 (d, J = 10.80Hz, 2H), 7.54 (d, J = 7.20Hz, 4H), 7.45 (t, J 1 =8.64Hz,J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com