Amphiphilic polymer nano micelle containing ferric ions chelated with polydopa amino acid and application thereof
An amphiphilic polymer and polydopa amino acid technology, which is applied in the field of amphiphilic polymer nanomicelles, can solve the problems of indistinguishable signal substances, restricted reticuloendothelial system, time-consuming and high cost, and achieves relaxation performance. High, obvious imaging effect, the effect of broadening the scope of medical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) The structural formula of polydopa amino acid-polysarcosine block copolymer (PDOPA-b-PSar) is as follows:
[0052]
[0053] Among them, R 1 Be benzyl; m=5~200, n=5~50;
[0054] Concrete synthetic steps include:
[0055] Add sarcosine NCA to the Schlenk bottle, dissolve it with DMF, then add the DMF solution of benzylamine, the molar ratio of sarcosine NCA to benzylamine is 5-200:1, react at room temperature for 1 day, then add benzyloxycarbonyl ( cbz) protected DMF solution of dopa NCA, the molar ratio of dopa NCA to benzylamine is 5-50: 1, react at room temperature for 1 day, pour the polymer solution into ether to precipitate, filter, and vacuum-dry the obtained polymer In 1 day, a cbz-protected polydopa amino acid-polysarcosine block copolymer was obtained.
[0056] Dissolve 300 mg of block copolymer in 3 mL of trifluoroacetic acid, add 4 times the equivalent of hydrogen bromide in acetic acid solution (33%), react for 3 hours, precipitate with ether, filte...
Embodiment 2
[0063] (1) Other preparation conditions are the same as in Example 1, except that polyethylene glycol amine is used as a macroinitiator, and the prepared polydopa amino acid-polyethylene glycol block copolymer (PDOPA-b-PEG ) has the following structural formula:
[0064]
[0065] Among them, R 2 It is methyl; m=5~200, n=5~50.
[0066] (2) Dissolve the weighed 9.7mg PDOPA-b-PEG in DMF to form a solution, then slowly add 3.27mg Fe(NO 3 ) 3 9H 2 O in DMF and dialyzed against deionized water for 48 hours. The obtained micellar solution was filtered with a filter membrane with a pore size of 0.45 μm and used at constant volume.
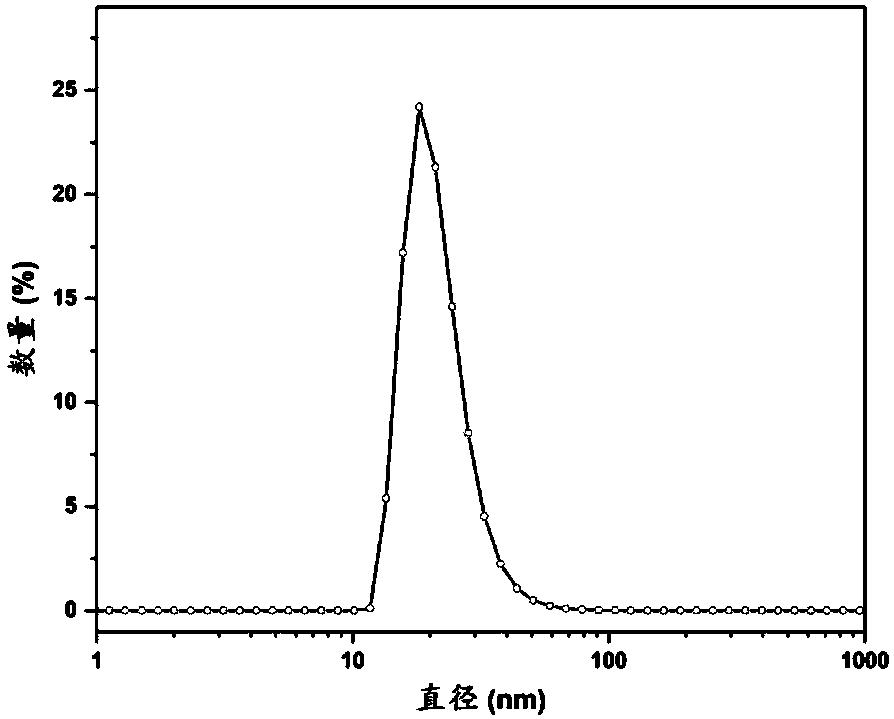
[0067] Other performance test conditions are the same as in Example 1, the average particle size of the micelles is 30nm, and it has MRI enhancing effect in vitro.
Embodiment 3
[0069] (1) The structural formula of polydopa amino acid-polyethylene glycol methacrylate graft polymer (POEGMA-g-PDOPA) is as follows:
[0070]
[0071] Among them, R 1 It is n-butyl; m=5~200, n 1 =5~50;
[0072] Concrete synthetic steps include:
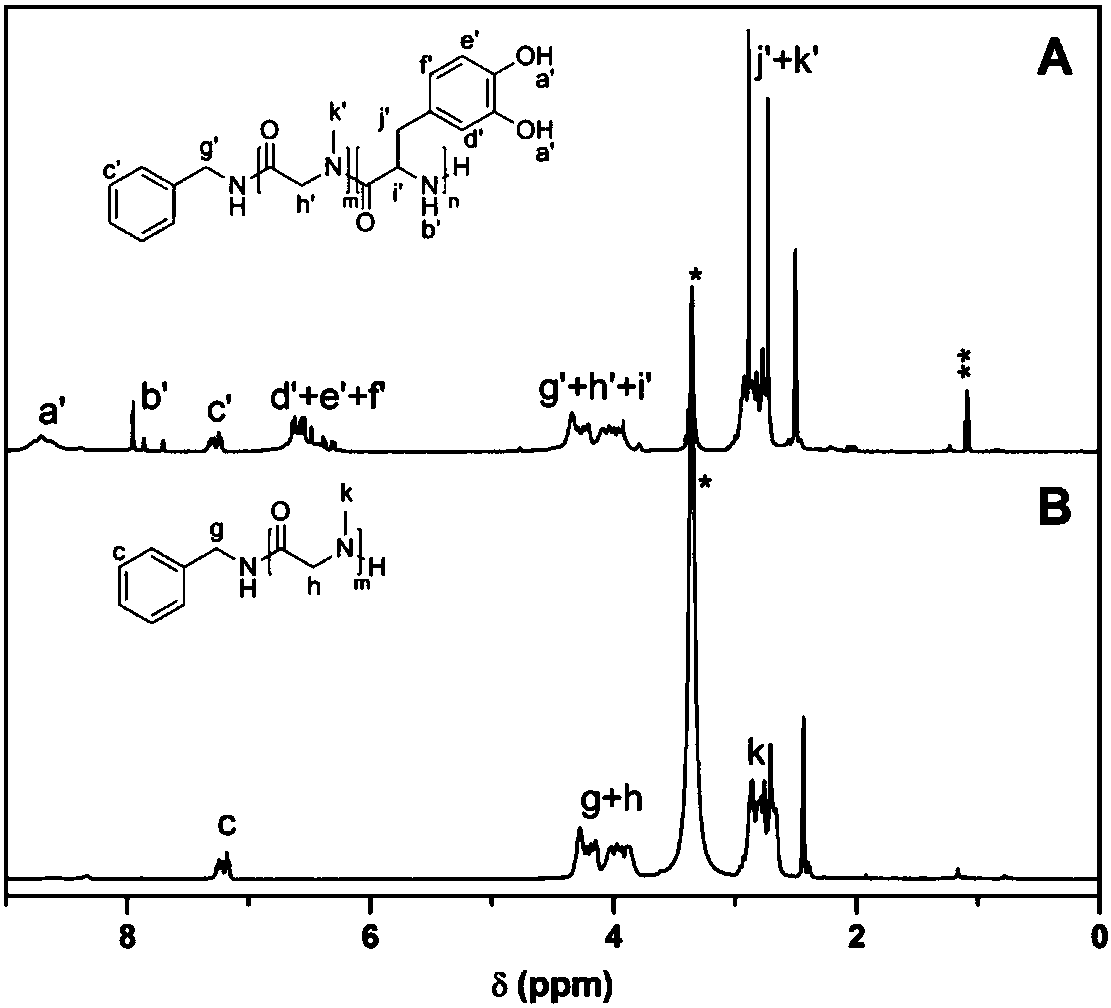
[0073] PDOPA was prepared by ring-opening polymerization and deprotection of cbz-protected dopa NCA initiated by n-butylamine, and the conditions were the same as in Example 1; poly(polyethylene glycol methacrylate) (POEGMA) was prepared by RAFT polymerization. Dissolve 247.4mg POEGMA and 134.0mg PDOPA in 1mL DMF, place in 35oC oil bath to react for 4 days, pour the polymer solution into ether to precipitate, filter, and vacuum dry for 1 day to obtain polydopa amino acid-polymethacrylic acid Oligoethylene glycol ester graft polymer. The H NMR spectrum of the polymer is shown in Figure 7 shown.
[0074] (2) Dissolve the weighed 22.7mg POEGMA-g-PDOPA in DMF to form a solution, then slowly add 5.83mg Fe(NO 3 ) 3 9H 2 O in D...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com