Synthesis method of doxylamine succinate
A technology of doxylamine succinate and synthesis method, which is applied in the field of synthesis of doxylamine succinate, can solve the problems of reaction equipment damage, long reaction time, and high cost of doxylamine, and achieve anticholinergic sedation effect, low gastrointestinal side effects, significant sedative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
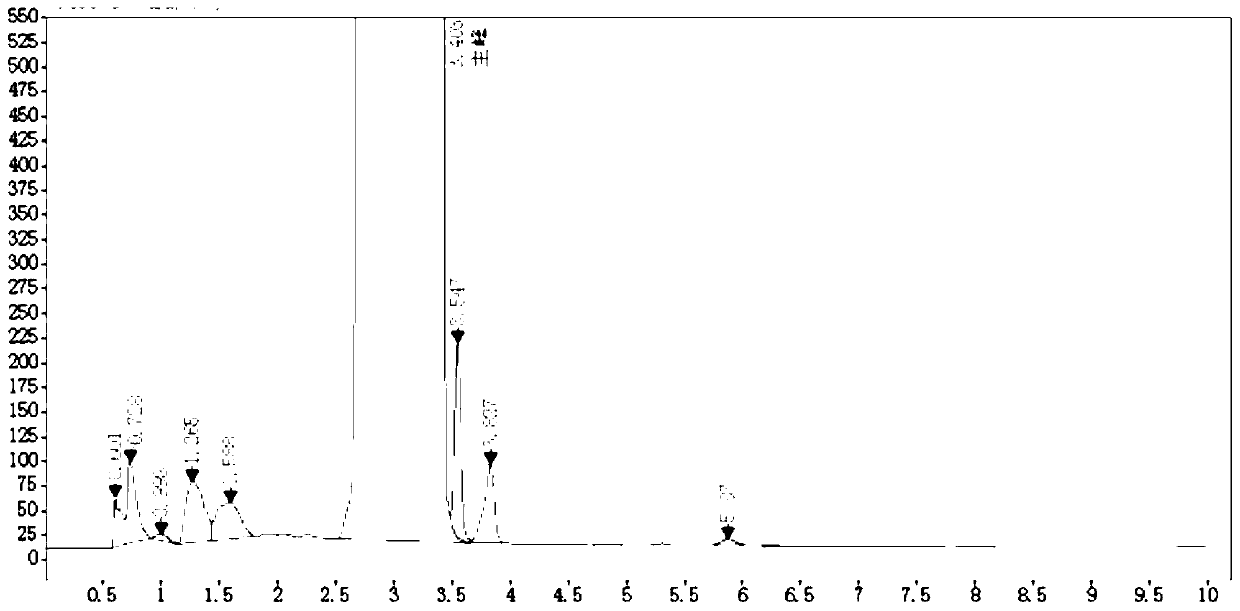
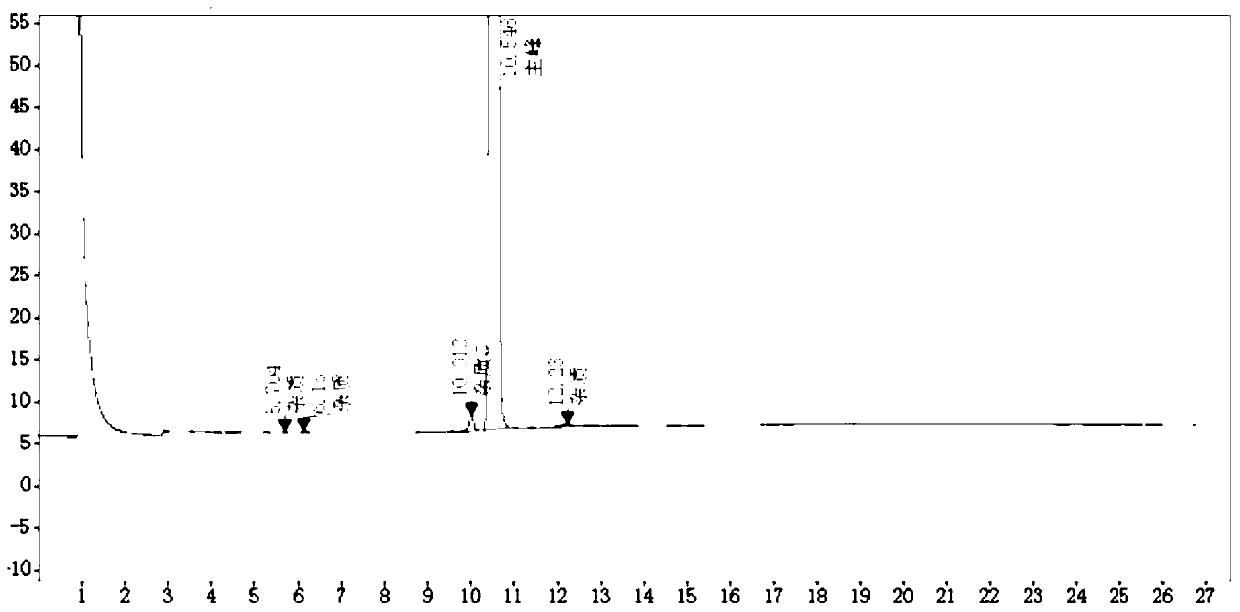
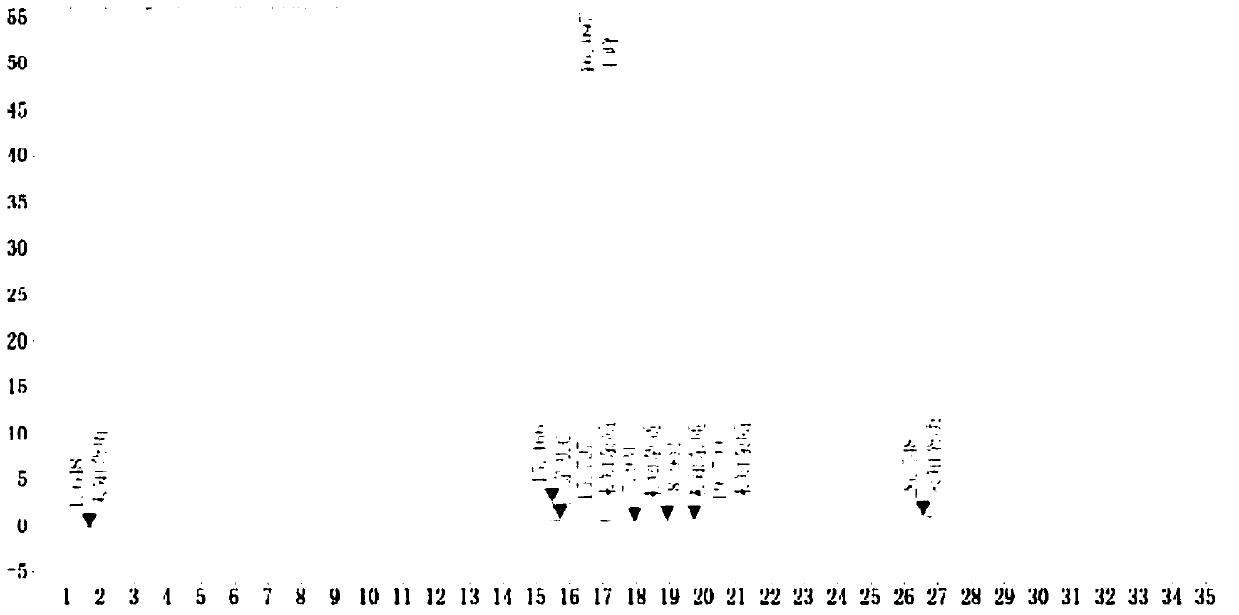
Image
Examples
Embodiment 1
[0057] (1) Put 500g of xylene into a 2000ml three-necked flask, add 100g (0.5mmol) of 2-pyridylphenylmethylmethanol, add catalyst tetrabutylammonium bromide 1g, sodium hydride 24.1g (0.6mmol) and 2 -Dimethylaminoethyl chloride 81g (0.75mmol), warming up to 108 ° C for reflux reaction for 2 hours, the reaction is complete; then cool down, filter, filter out the solid and collect the filtrate, add 20wt% hydrochloric acid aqueous solution to make the filtrate pH=3.5, stir After 30 minutes, separate the aqueous phase and the organic phase, continue to add 50g of 20wt% hydrochloric acid aqueous solution to the organic phase, stir after 30 minutes, separate the aqueous phase, merge the aqueous phase twice, and backwash twice with xylene 50g each time. Add activated carbon and raise the temperature to 75°C for decolorization for 1 hour and then filter the filtrate. Cool the filtrate to 15°C and add 40wt% sodium hydroxide to make the pH 11.5, then add 100 g of n-hexane each time to ext...
Embodiment 2
[0060] (1) Add 700g of toluene into a 2000ml three-necked flask, add 100g (0.5mmol) of 2-pyridylphenylmethylmethanol, and add 2g of catalyst tetrabutylammonium iodide, 1.2mmol of sodium tert-butoxide and 2-dimethyl Aminochloroethane 64.8g (0.6mmol), heat up to 105°C for reflux reaction for 1.8 hours, the reaction is complete; then cool down, filter, filter out the solid and collect the filtrate, add 15wt% hydrochloric acid aqueous solution to make the filtrate pH = 3, stir for 30 minutes Finally, separate the water phase and the organic phase, continue to add 15wt% hydrochloric acid aqueous solution to the organic phase to make pH=3, after stirring for 30 minutes, separate the water phase, combine the water phase twice, and then backwash twice with toluene 50g each time, add Activated carbon was heated up to 78°C for decolorization for 1 hour and then filtered, and the filtrate was cooled to 20°C and added with 40wt% sodium hydroxide to make the pH 11, then 100 g of ether was a...
Embodiment 3
[0063] (1) Add 600g of toluene into a 2000ml three-necked flask, add 100g (0.5mmol) of 2-pyridylphenylmethylmethanol, add catalyst tetrabutylammonium bromide 3g, metal potassium 0.6mmol and 2-dimethylamino Ethyl chloride (1mmol), warming up to 110°C for reflux reaction for 1.6 hours, the reaction is complete; then cool down, filter, filter out the solid and collect the filtrate, add 25wt% hydrochloric acid aqueous solution to make the filtrate pH=4, after stirring for 30 minutes, separate the water phase And the organic phase, continue to add 25wt% hydrochloric acid aqueous solution to the organic phase, after stirring for 30 minutes, separate the water phase, combine the water phase twice, and then backwash twice with toluene 80g each time, add activated carbon and heat up to 76 ° C for 1 hour decolorization Filtrate, cool the filtrate to 22°C and add 45wt% sodium hydroxide to make the pH 12, add 100g of dichloromethane each time to extract the aqueous phase 3 times, combine t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com