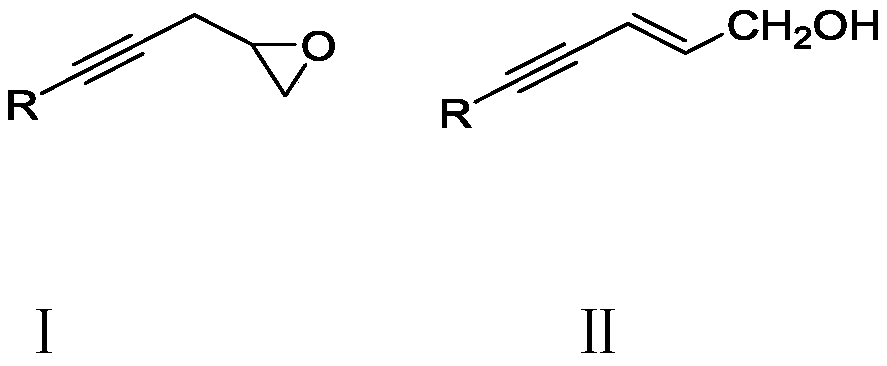
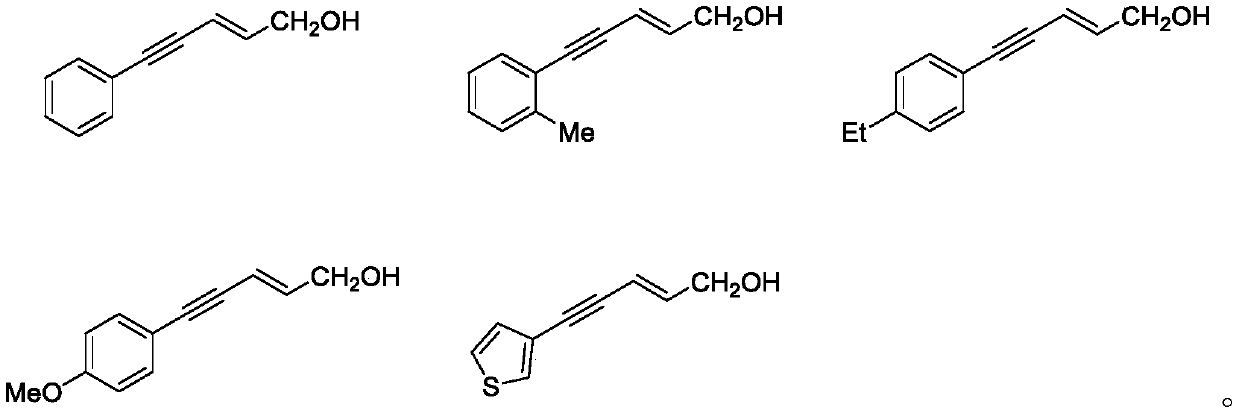
Method for synthesizing trans-2-alkene-4-alkyne-1-alcohol compound
A compound and alcohol technology, applied in the field of organic compound synthesis, can solve the problems of single space structure, difficult product, high toxicity of propynyl alcohol, etc., and achieve the effect of good selectivity, single structure and simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
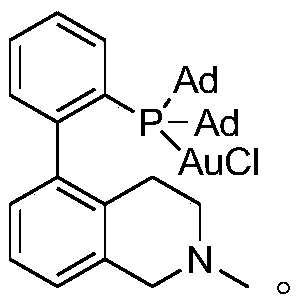
[0030] The preparation process of gold catalyst described in the embodiment is:
[0031] Synthesis of A: Add 5-bromoisoquinoline (10mmol), 2-bromophenylboronic acid (1.06equiv.), tetrakis(triphenylphosphine) palladium (5mol%), carbonic acid Sodium hydrogen (4.5 equiv.), and under nitrogen protection, add 20ml of ethylene glycol dimethyl ether and 10ml of deionized water into the bottle with a syringe, and heat the system to 95°C to react overnight. After the reaction, cool to room temperature, extract with dichloromethane, collect the organic phase, and extract the water phase twice, combine the organic phase, wash the organic phase with water, and dry with anhydrous sodium sulfate, then use column chromatography Product A was isolated.
[0032] Synthesis of B: Add A (10 mmol) and 30 ml tetrahydrofuran into a round-bottomed flask equipped with a magnet, add methyl iodide (1.5 equiv.) under stirring at room temperature, and react for 24 hours. After the reaction, a large numb...
Embodiment 1
[0039]
[0040] 0.004mmol Add 0.02mmol tetrakis(3,5-bis(trifluoromethyl)phenyl)sodium borate into a 15ml Schlenck tube, after the nitrogen protection is completed, add 0.2mmol 2-(3-phenyl-2-propynyl) with a syringe Ethylene oxide and 2 mL of DCE. Next, the reaction was carried out in a 90° C. oil bath for 12 hours. After the reaction finished, add two spoonfuls of column chromatography silica gel (100-200 mesh) in the reaction solution, and remove the solvent by distillation under reduced pressure, then obtain the pure product by column chromatography (with sherwood oil / ethyl acetate=5 :1 as eluent). The material was a yellow liquid and the yield was 68%.
[0041] Characterization data: 1 H NMR (500MHz, CDCl3) δ7.46-7.44 (m, 2H), 7.34-7.32 (m, 3H), 6.37 (dt, J = 15.9, 5.2Hz, 1H), 5.99 (dt, J = 15.9, 1.8 Hz,1H), 4.29(dd,J=5.2,1.8Hz,2H),1.66(s,1H).; 13 C NMR (125MHz, CDCl 3 ) δ141.7, 131.5, 128.3, 128.2, 123.2, 110.5, 90.2, 87.2, 63.1.
Embodiment 2
[0043]
[0044] 0.01mmol Add 0.02mmol tetrakis(3,5-bis(trifluoromethyl)phenyl)sodium borate into a 15ml Schlenck tube, after the nitrogen protection is completed, add 0.2mmol 2-(3-(o-methyl)phenyl- 2-propynyl)oxirane and 2mL DCE. Next, the reaction was carried out overnight in an oil bath at 90°C. After the reaction finished, add two spoonfuls of column chromatography silica gel (100-200 mesh) in the reaction solution, and remove the solvent by distillation under reduced pressure, then obtain the pure product by column chromatography (with sherwood oil / ethyl acetate=5 :1 as eluent). The material was a yellow liquid, 65% yield.
[0045] Characterization data: 1 H NMR (500MHz, CDCl3) δ7.42 (d, J = 7.5Hz, 1H), 7.23–7.20 (m, 2H), 7.17–7.13 (m, 1H), 6.36 (dt, J = 15.9, 5.3Hz, 1H), 6.03(dt, J=15.9, 1.8Hz, 1H), 4.29(dd, J=5.3, 1.8Hz, 2H), 2.45(s, 3H), 1.63(s, 1H); 13 C NMR (125MHz, CDCl3) δ1 41.3, 140.1, 131.8, 129.4, 128.2, 125.5, 123.0, 110.8, 91.1, 89.1, 63.0, 20.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com