Oxidative stress drug system with charge reversal capability and preparation method of oxidative stress drug system
A technology of oxidative stress and charge reversal, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc. Cell level, affecting life activities and other issues, to achieve the effect of improving utilization rate and therapeutic effect, enhancing oxidative stress, and enhancing encapsulation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The preparation method of the oxidative stress drug system drug carrier system with charge reversal ability of the present invention mainly includes the following steps:
[0038] Step 1, Synthesis of Hollow Manganese Dioxide HMDN
[0039] (1) Quickly add 2.0~4.0mL TEOS (tetraethyl orthosilicate) to a mixed solution containing 30~60mL ethanol, 30~60mL deionized water and 1~6mL ammonia water (25%-28%), at room temperature Stir for 0.2 to 1 hour to form white colloidal suspended solid SiO 2 , and the SiO was cleaned by centrifugation with deionized water and ethanol 2 (alcohol twice / water twice, lyophilization without organic solvents) lyophilization to obtain sSiO 2 ;
[0040] (2) 180~300mg sSiO2 2 Fully disperse in deionized water, add this suspension to the mixed solution containing 500-800mg CTAB (remove 38-62mL deionized water, 38-62mL ethanol and 1-6mL ammonia water); stir the obtained mixed solution at room temperature for 0.2-1h , 400-700 mg KMnO4 was added qu...
Embodiment 1
[0051] A preparation method of an oxidative stress drug system with charge reversal ability, specifically comprising the following steps:
[0052] 1. Preparation of hollow manganese dioxide HMDN
[0053] (1) Quickly add 3.0mL TEOS to a mixed solution containing 37mL ethanol, 5mL deionized water, and 1.6mL ammonia water (ammonia water concentration 25%-28%), stir at room temperature for 0.5h, and form a white colloidal suspended solid SiO 2 , and the SiO was cleaned by centrifugation with deionized water and ethanol 2 (alcohol twice / water twice, freeze-drying without organic solvents), freeze-drying to obtain sSiO 2 ;
[0054] (2) 200mg sSiO2 was fully dispersed in 40mL deionized water, and then the suspension was added to the mixed solution containing 600mg CTAB (the mixed solution was composed of 60mL deionized water, 60mL ethanol and 4.5mL ammonia water, then stirred at room temperature for 0.5 h, quickly add 600mg KMnO 4 Then continue to react for 6h, collect by centri...
Embodiment 2
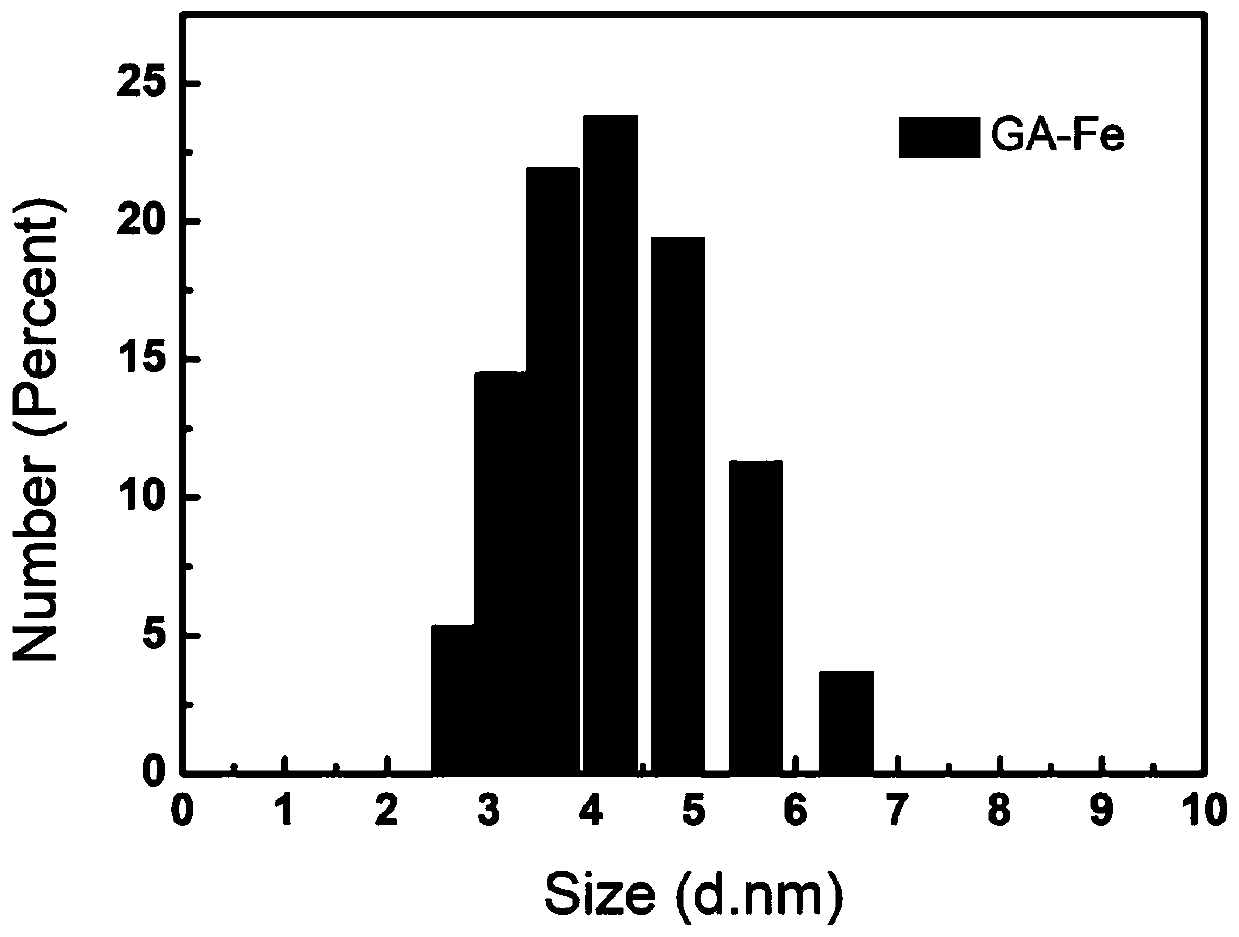
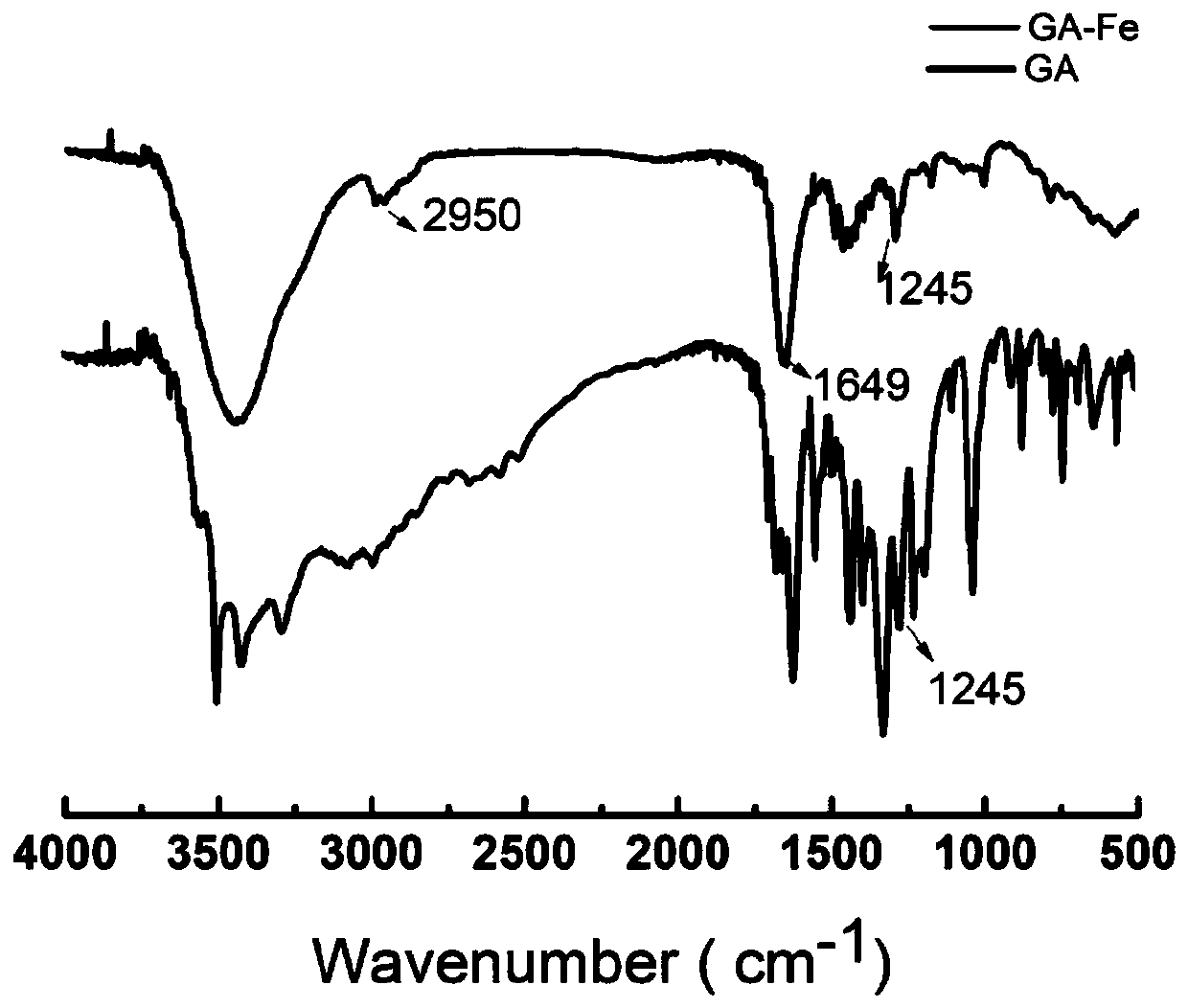
[0073] The preparation method of an oxidative stress drug system with charge reversal ability specifically includes the following steps: Weigh 200 mg of HMDN and dissolve it in 100 mL of tris (pH 8.0), add 200 mg of GA-Fe, and store under nitrogen protection at 25 to 35 ° C. Stir lightly for 24 hours, then add 100ml of tris solution (30mg / ml, pH 7.4) dropwise with PEI and react for 2 hours, finally add 200mg PASP-API and react for 24 hours, then wash with tris and deionized water and lyophilize.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com