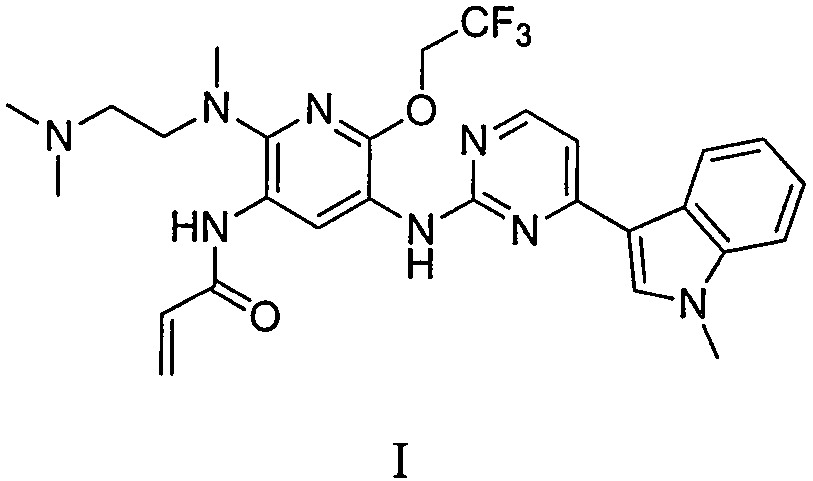
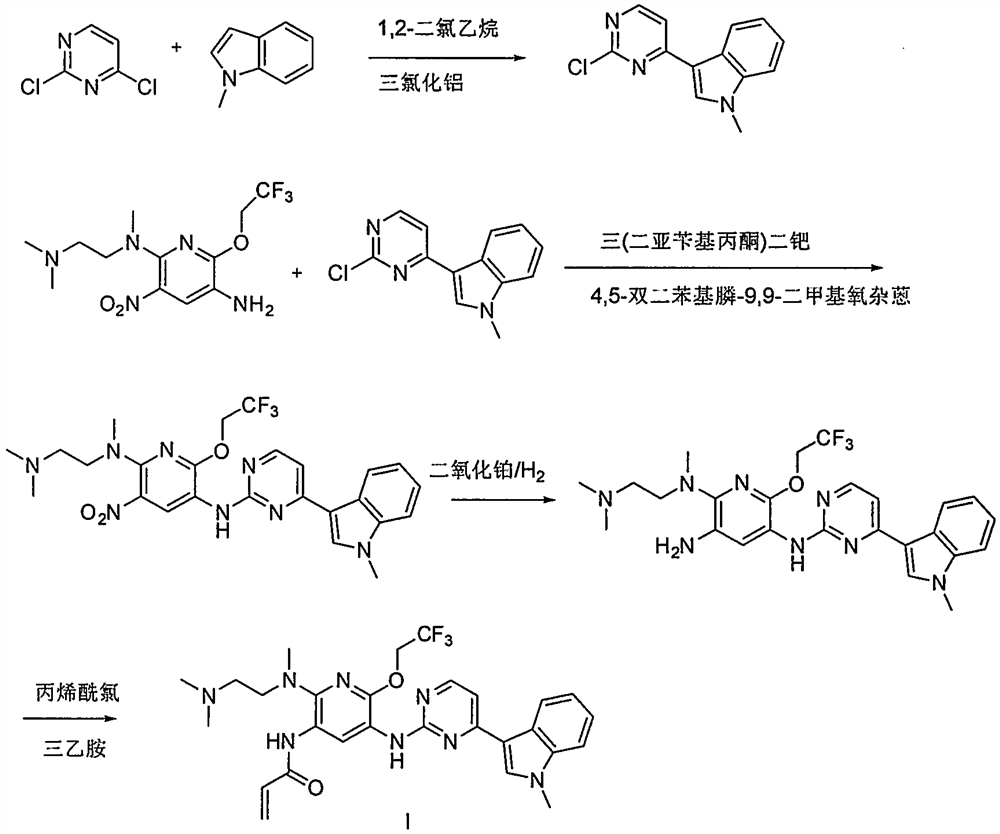
Preparation method and intermediate of pyridineaminopyrimidine derivatives
A pyrimidine and amine-based technology is applied in the fields of organic synthesis and the preparation of raw materials, which can solve the problems of many steps in the overall preparation route, unsuitable for industrial production, troublesome post-processing, etc., achieves low requirements for reaction equipment, promotes complete reduction reaction, The effect of improving the reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
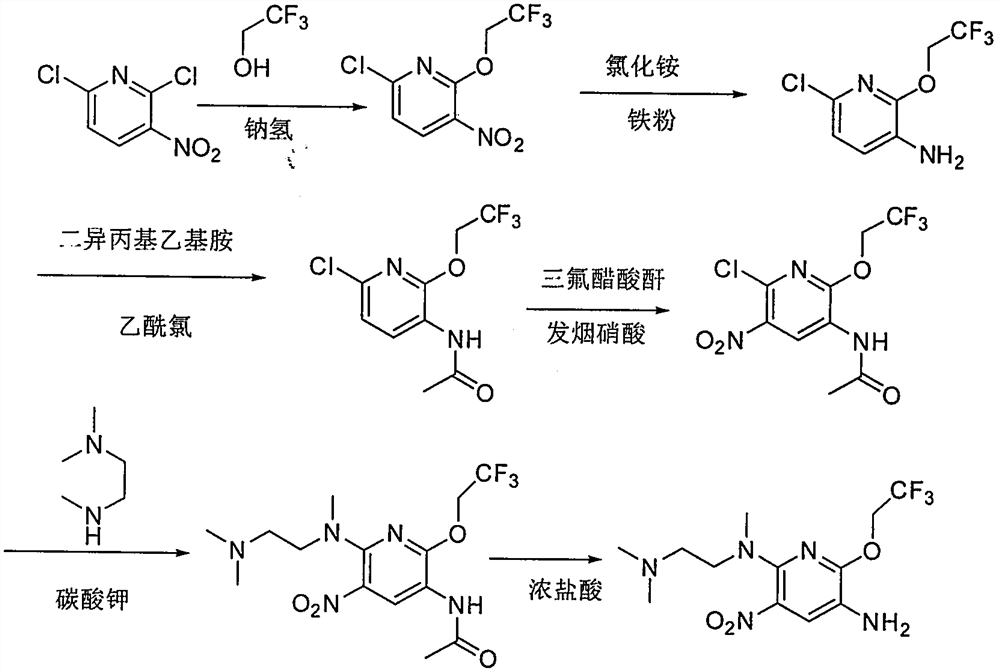
[0138] Example 1: Preparation of 6-chloro-3-nitro-2-(2,2,2-trifluoroethoxy)pyridine (XI-1)
[0139] Add toluene (24.0L) to the reaction kettle, then add 2,6-dichloro-3-nitropyridine (3000g, 15.54mol), adjust the internal temperature between -20°C and -10°C, and add sodium in batches Hydrogen (933 g, 23.33 mol). A solution of 2,2,2-trifluoroethanol (1586 g, 16.00 mol) in toluene (6.0 L) was added dropwise. After 2 hours of reaction, TLC and HPLC monitored the end point of the reaction. After the reaction was complete, 10% ammonium chloride solution (6.0 L) was added dropwise. Let stand, layer. The organic phase was washed with water (6.0 L) and concentrated under reduced pressure. Add ethyl acetate (0.3L), heat up to 40-50°C, add n-heptane (2.7L) dropwise, cool down to -15 to -5°C to continue crystallization for 3 hours, and filter with suction. 3017 g of product solids were obtained with a yield of 75.65%.
[0140] 1 H NMR (500MHz, DMSO-d6) δ8.60 (d, J = 8.0Hz, 1H), 7.5...
Embodiment 2
[0143] Example 2: Preparation of 6-chloro-3-amino-2-(2,2,2-trifluoroethoxy)pyridine (X-1)
[0144] At room temperature, add acetonitrile (21.0L) and water (21.0L) to the reaction kettle, start stirring, and add the 6-chloro-3-nitro-2-(2,2,2-trifluoroethane obtained in Example 1 Oxy)pyridine (3017.0g, 11.76mol), add hydrosulfite (15.1Kg, 70.54mol). React for 2 hours under the condition of controlling the temperature at 27-33°C. 36% concentrated hydrochloric acid (11.9Kg, 117.60mol) was added dropwise, and the reaction was continued for 1.5 hours. Solid sodium bicarbonate (12.8 Kg, 12.96 mol) was added. After filtration, the mother liquor was separated into layers, and the organic phase was washed with saturated brine (21.0 L), and concentrated under reduced pressure to obtain an oily substance.
[0145] 1 H NMR (500MHz, DMSO-d6) δ7.03(d, J=8.0Hz, 1H), 6.90(d, J=8.0Hz, 1H), 5.21(s, 2H), 4.93(q, J=9.0Hz ,2H);
[0146] 13 C NMR (126MHz, DMSO-d6) δ 148.16, 131.72, 130.55, 12...
Embodiment 3
[0148] Example 3: Preparation of 6-chloro-3-(2,2,2-trifluoroacetamido)-2-(2,2,2-trifluoroethoxy)pyridine (IX-1)
[0149] At room temperature, dichloromethane (10.4 L) was added to the reaction kettle, stirring was started, and 6-chloro-3-amino-2-(2,2,2-trifluoroethoxy)pyridine (2664 g , 11.76mol), diisopropylethylamine (2279g, 17.64mol) was added, the temperature was controlled from -15 to -10°C, and a solution of trifluoroacetic anhydride (2963g, 14.11mol) in dichloromethane (5.2L) was added dropwise After dripping, continue to stir for 20 minutes. Water (13.0L) was added dropwise, the layers were separated, the organic phase was concentrated under reduced pressure, and the theoretical calculation was carried out to the next step reaction.
[0150] 1 H NMR (400MHz, DMSO-d6) δ11.23(s, 7H), 7.95(d, J=8.0Hz, 1H), 7.34(d, J=8.0Hz, 1H), 5.03(q, J=8.9Hz ,2H);
[0151]13 C NMR (101MHz, DMSO-d6) δ155.74(q, J=46.6Hz), 155.60, 145.37, 140.24, 124.01(q, J=278.8Hz), 119.07, 118.30, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com