Inhibitor for monoamine oxidase A
A monoamine oxidase and inhibitor technology, applied in the field of monoamine oxidase A inhibitor and its synthesis, can solve the problems of ruthenium tetroxide instability, high price, high toxicity, etc., achieve good monoamine oxidase A inhibitory activity, simple operation process, and subsequent treatment simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
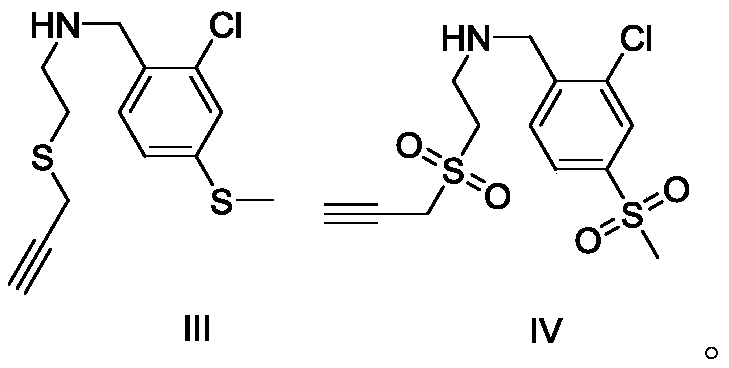
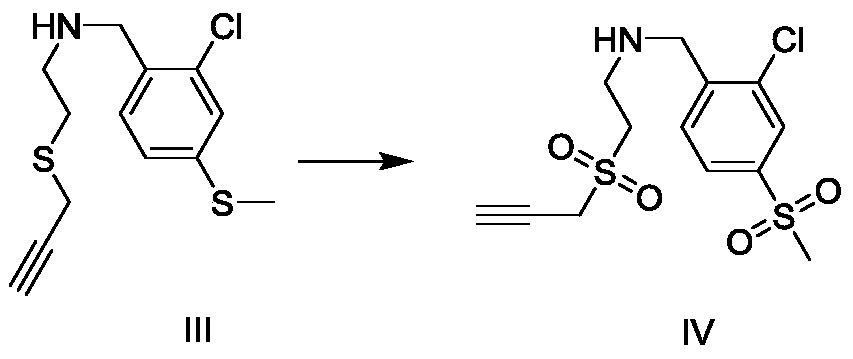
[0023] Embodiment 1: the preparation of ether compound III
[0024] The reaction formula is as follows:
[0025]
[0026] In the reaction flask, add 1.258g (5.0mmol) compound II (purchased from Sigma-Aldrich company) and 1.382g (10.0mmol) potassium carbonate, dissolve with 60mL acetone, start stirring, then add 0.576g (5.0mmol) compound therein I (purchased from Sigma-Aldrich Company), heated and refluxed for 7 hours. The TLC method was used to detect that the reaction of compound II was complete, and the reaction was stopped. The reaction liquid was filtered and washed with dichloromethane (50 mL×2), and the filtrate and washing liquid were combined. Washed twice with saturated sodium chloride solution, dried with anhydrous sodium sulfate, evaporated to remove the solvent, and separated by column chromatography (mobile phase n-hexane: ethyl acetate = 15:1) to obtain the refined compound III with a yield of 77% . The structural representation of compound formula III is ...
Embodiment 2
[0028] Embodiment 2: the preparation of sulfone compound IV
[0029] The reaction formula is as follows:
[0030]
[0031] Add 0.02g Ru / C catalyst, sodium periodate 470mg (2.2mmol) in the reaction bottle, then add 4mL water, then add 285mg (1mmol) compound III in the reaction bottle under stirring state, react at room temperature 2h. The TLC method was used to detect that the reaction of compound III was complete, and the reaction was stopped. The reaction liquid was filtered and washed with dichloromethane (15 mL×2), and the filtrate and washing liquid were combined. Wash twice with saturated sodium chloride solution, dry with anhydrous sodium sulfate, and evaporate the solvent to obtain compound IV with a yield of 99%. The structural representation of compound formula IV is as follows:
[0032] 1 H NMR (500MHz, CDCl 3 )δ8.29-7.60(m,3H),4.27(s,1H),4.20-4.17(m,2H),3.92-3.88(m,2H),3.56-3.53(m,2H),3.27-3.24( m,2H),3.19(s,1H),3.34(s,3H). 13 C NMR (125MHz, CDCl 3 )δ141...
Embodiment 3
[0033] Example 3: Monoamine Oxidase A Inhibitory Activity Test
[0034] (1) Sample preparation
[0035] The compound (IV) prepared in Example 2 was dissolved in dimethyl sulfoxide (DMSO), and prepared into samples with a concentration gradient of 5, 15, 25, 35, 45, 60, 75, 90, 105, and 120 mmol / L respectively liquid, denoted as sample 1.
[0036] (2) Compound (IV) test method for monoamine oxidase-A inhibitory activity
[0037] Add 4 μL of monoamine oxidase-A (MAO-A) and 4 μL of sample 1 prepared in step (1) to EP tubes containing 386 μL of boric acid buffer (pH=8.4), mix, and react the mixture in a water bath at 38 °C for 2 h , and then add 2 μL of probe 7-(3-aminopropoxy)-4-methylcoumarin (10 mmol / ml) and 4 μL of bovine serum albumin ( BSA), and the EP tube was placed in a 38°C water bath to continue the reaction for 2h. At the same time, it is necessary to detect the enzyme activity of the enzyme without inhibitor, that is, add 4 μL monoamine oxidase-A (MAO-A) to the EP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com