Method for preparing cefoperazone deuterated internal standard substance
A technology of cefoperazone and internal standard, which is applied in the field of preparation of cefoperazone deuterated internal standard, can solve problems such as poor effect, and achieve the effect of controllable experimental process, high purity and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
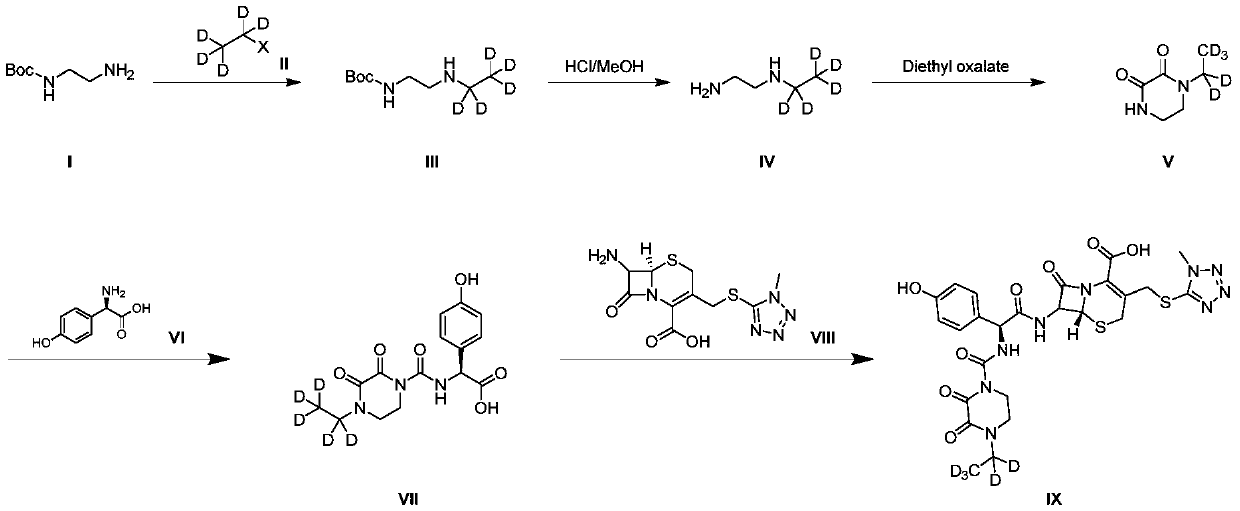
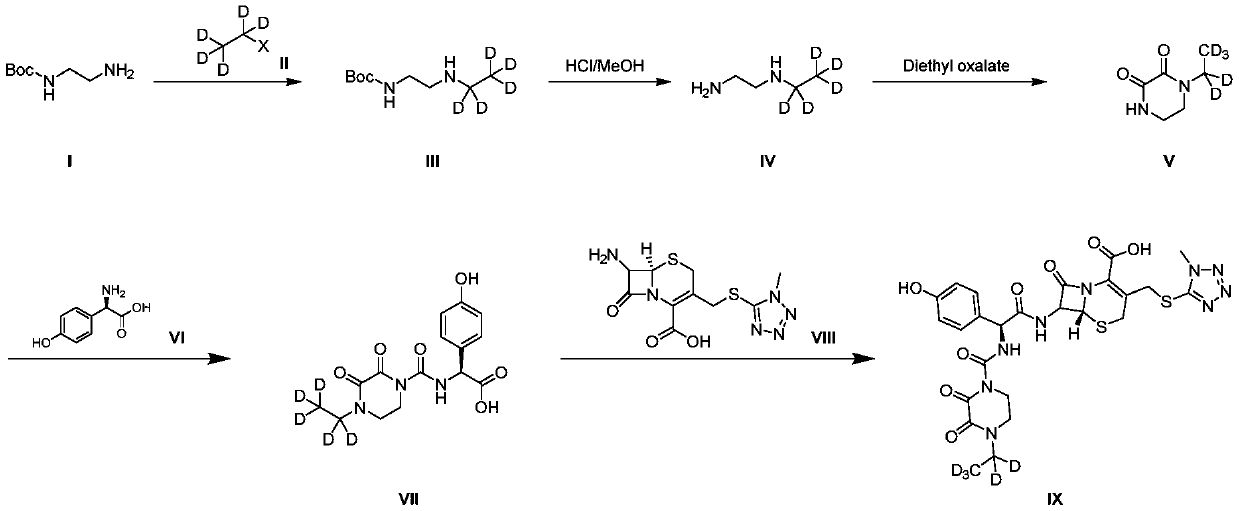
[0041] Synthesis of N-Boc-N'-ethylethylenediamine-D5(III)
[0042] Boc-ethylenediamine (1g) and triethylamine (1.8mL, 2.0eq) were dissolved in tetrahydrofuran (10mL), cooled to 0-5°C, and deuterobromoethane ( 0.7g, 1.0eq), the addition was completed, and the temperature was slowly raised to room temperature for 15h. The reaction was monitored by TLC (DCM:MeOH=10:1). After the reaction was completed, it was diluted with water, extracted with ethyl acetate, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated in vacuo to obtain the crude product, which was used on a FLASH column Purified by chromatography to obtain N-Boc-N'-ethylethylenediamine-D5(III) (0.65g) with a yield of 54.1%. LCMS (ESI+): m / z = 194.2 [M+H]+.
Embodiment 2
[0044] Synthesis of N-Boc-N'-ethylethylenediamine-D5(III)
[0045] The raw materials Boc-ethylenediamine (1g), p-toluenesulfonic acid (D5-ethyl) ester (1.3g, 1.0eq) were dissolved in acetonitrile (10mL), potassium carbonate (0.9g, 1.5eq) was added, and the reaction mixture Put it in an oil bath at 85°C and heat it for 14 hours. After the reaction, add 20mL of water and 50mL of ethyl acetate, separate the organic layer, concentrate under reduced pressure to obtain a crude product, and purify it by FLASH column chromatography to obtain N-Boc-N' -Ethylethylenediamine-D5(III) (0.7g), yield 58.3%. LCMS (ESI+): m / z = 194.2 [M+H]+.
[0046] 1H NMR (400MHz, Chloroform-d) δ4.99(s, 1H), 3.23(q, J=5.9Hz, 2H), 2.74(t, J=5.9Hz, 2H), 1.44(s, 9H).
Embodiment 3
[0048] Synthesis of N-ethyl-2,3-diketopiperazine-D5(V)
[0049] Intermediate (III) (0.5 g) was dissolved in methanolic hydrochloric acid solution (3N, 20 mL, 40 V) under ice-cooling, slowly warmed to room temperature, and stirred for 5 h. After the reaction, it was concentrated to obtain 0.45 g of intermediate (IV).
[0050] Intermediate (IV) (0.45g) was suspended in ethanol (50mL), triethylamine (1mL, 2eq) was added at room temperature, and stirred for 30min. The ethanol solution of diethyl oxalate (0.4g, 1.0 eq), after addition, react at room temperature for 15h. TLC (DCM:MeOH=10:1) monitors the reaction. After the reaction is completed, the reaction mixture is concentrated to obtain a crude product, which is purified by FLASH column chromatography to obtain N-Boc-N'-ethylethylenediamine-D5 (0.3g), yield 75.0%. LCMS (ESI+): m / z = 148.1 [M+H]+.
[0051] 1H NMR (400MHz, Deuterium Oxide) δ3.69 (dd, J=7.4, 4.7Hz, 4H), 3.56 (dd, J=7.3, 4.7Hz, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com