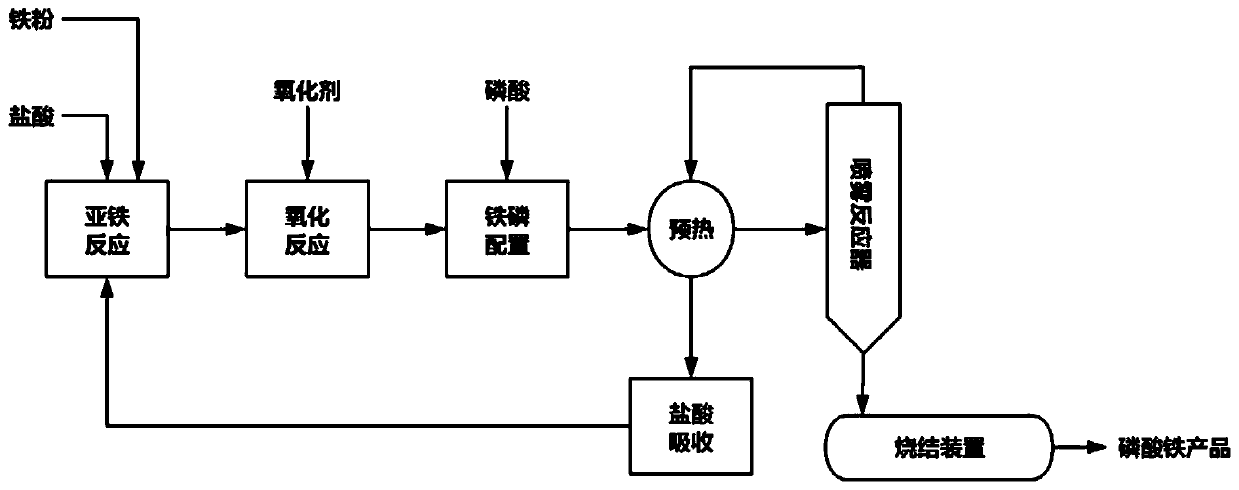
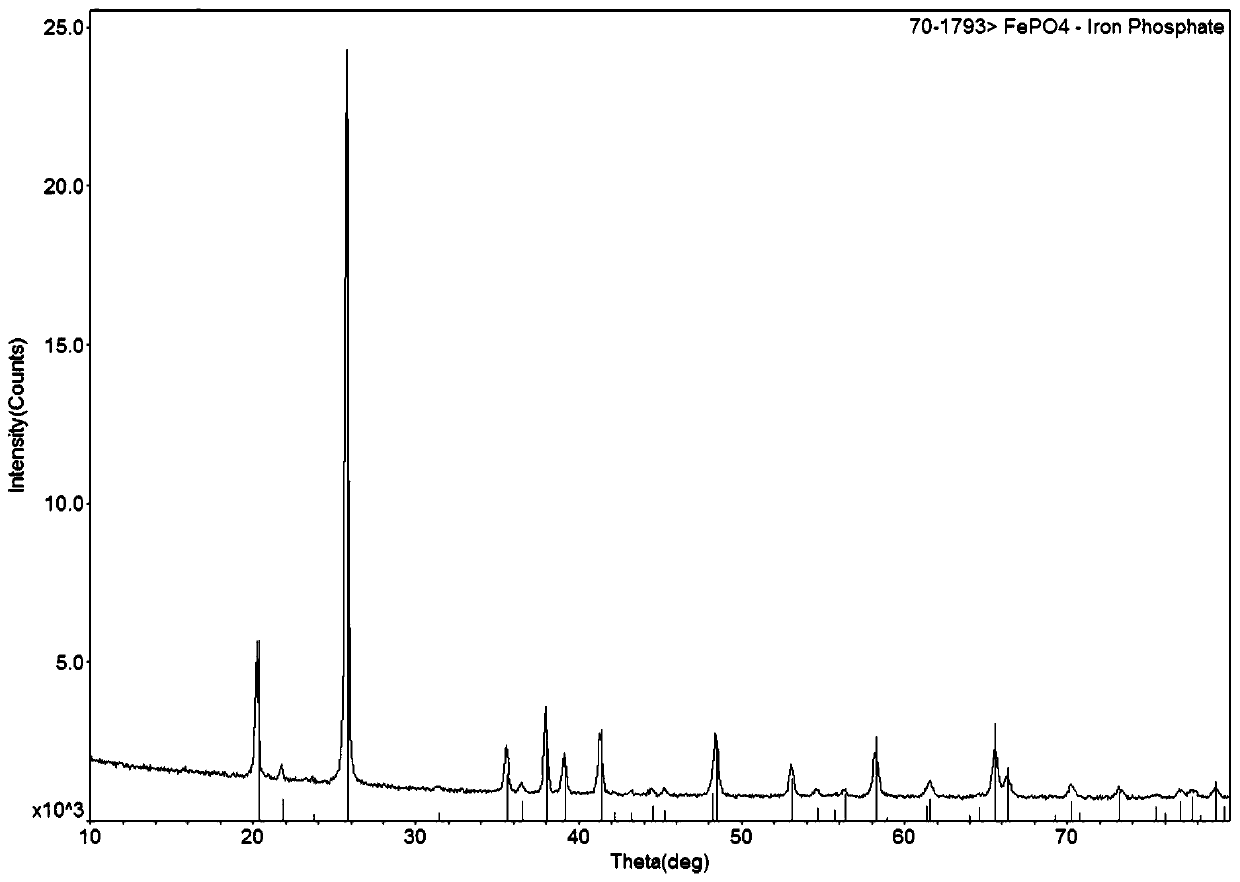
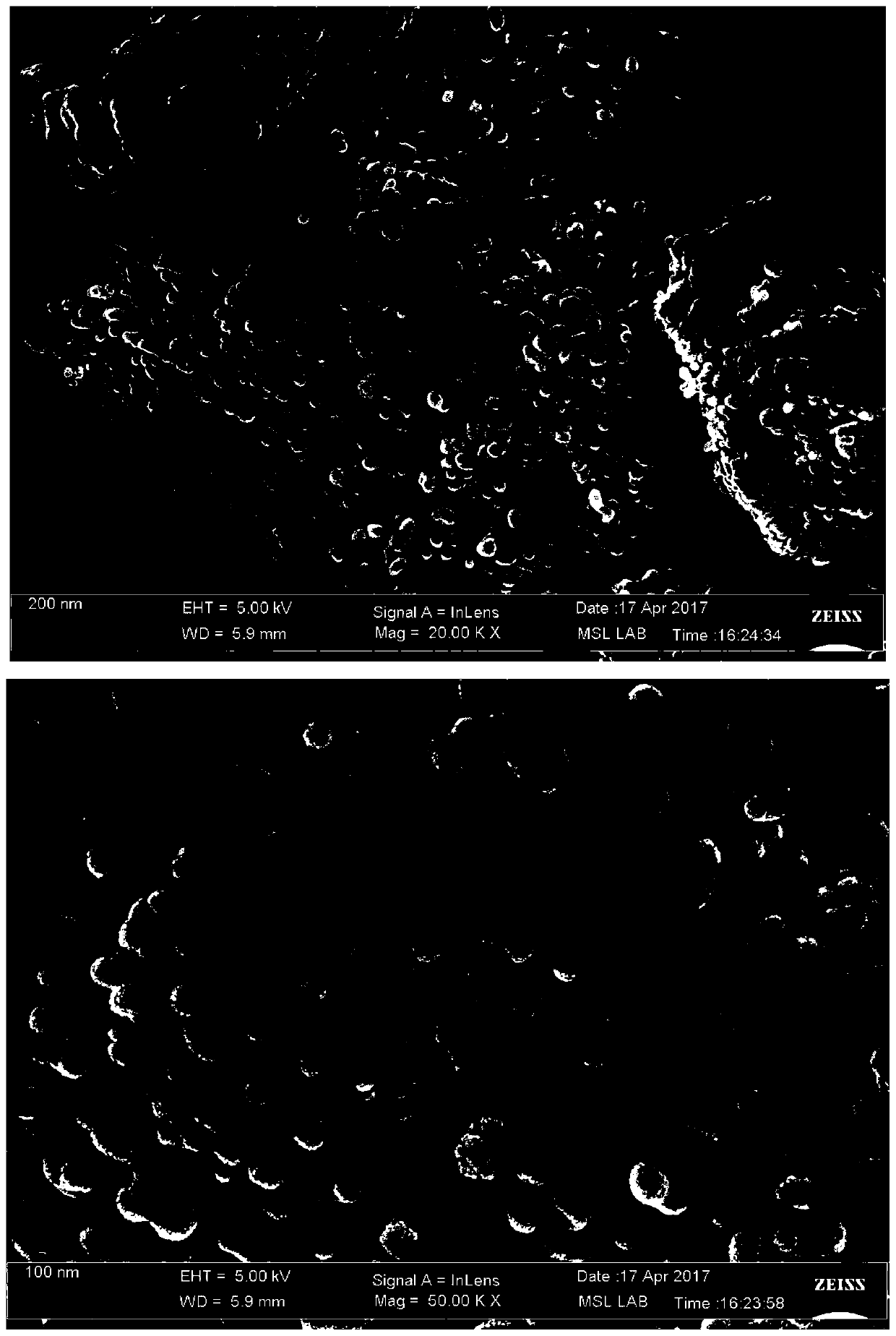
Preparation method of battery-grade iron phosphate
An iron phosphate, battery-grade technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of complex production process of iron phosphate, unreusable raw materials, high cost of sewage treatment, and improve resource utilization, The effect of reduced production cycle and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Concentrated hydrochloric acid with a concentration of 37% was prepared with deionized water to form 547.5kg of a 20% solution, and then 57.14kg of high-purity iron powder with a fineness of 100 mesh and a purity of 98% was added to fully react, and the reaction temperature was controlled to 65°C through jacket heat exchange , until the iron powder no longer dissolves.
[0040] After filtering, take the filtrate, add 74.18kg of 27.5% hydrogen peroxide, control the temperature at 55°C, and react for 3 hours, detect the absence of ferrous ions in the iron solution by the o-phenanthroline indicator method, and obtain the oxidizing solution.
[0041] Add 117kg of 85% phosphoric acid, mix evenly, and transport it to the spray dryer, control the air inlet temperature to 195°C, and the air outlet temperature to 105°C, and the solid collected after evaporation is ferric phosphate dihydrate, and the water vapor and hydrogen chloride condensate are recovered for storage spare.
...
Embodiment 2
[0046] Concentrated hydrochloric acid with a concentration of 37% was configured with deionized water to form 547.5kg of a 20% solution, and then 57.14kg of high-purity iron powder with a fineness of 100 mesh and a purity of 98% was added to fully react, and the reaction temperature was controlled by a heat exchanger to be 55 °C until no longer dissolved.
[0047]After filtering, take the filtrate, add 92.72 kg of 27.5% hydrogen peroxide, control the temperature at 65° C., react for 2 hours, detect the absence of ferrous ions in the iron solution by potassium permanganate titration, and obtain the oxidized solution.
[0048] Add 118.7kg of 85% phosphoric acid, mix well and transport to the spray dryer, control the inlet air temperature to 200°C, and the outlet air temperature to 105°C, and collect the solid obtained after evaporation as ferric phosphate dihydrate, recover water vapor and hydrogen chloride condensate for storage spare.
[0049] Send the ferric phosphate dihydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com