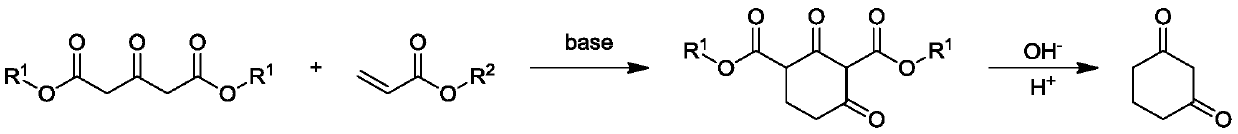
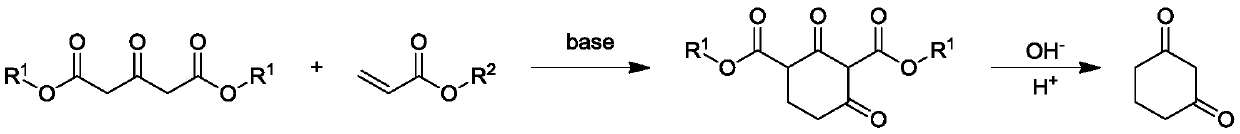
Preparation method of 1,3-cyclohexanedione
A technology of cyclohexanedione and acetone dicarboxylate, which is applied in the field of preparation of 1,3-cyclohexanedione, can solve problems such as low reaction conversion rate and yield, increased risk, and high environmental pressure, and achieves High production efficiency, low pollution, and high-efficiency synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A preparation method for 1,3-cyclohexanedione, comprising the steps of:
[0028] (1) Under the protection of nitrogen, add 250ml of anhydrous methanol to a 500ml three-necked flask equipped with a thermometer, reflux condenser and mechanical stirring, add 0.2mol of sodium methylate, stir to dissolve, and then add 1mol of 1,3-acetonedicarboxylic acid dimethyl ester, slowly add 1mol methyl acrylate dropwise, control the internal temperature at 25-30°C, stir at this temperature for 1h, then heat the reaction solution to 60°C, add 1.8mol sodium methoxide, stir for 4h, after the reaction is completed, concentrate and remove Anhydrous methanol, the residue is the intermediate;
[0029] (2) in the 1000ml there-necked flask that thermometer, reflux condenser and mechanical stirring are housed, add the sodium hydroxide aqueous solution of 400g mass concentration 20%, then add the intermediate that step (1) makes, be heated to 70-80 ℃, After 2 hours, add concentrated hydrochlori...
Embodiment 2
[0032] A preparation method for 1,3-cyclohexanedione, comprising the steps of:
[0033] (1) Under nitrogen protection, add 250ml of absolute ethanol to a 500ml three-necked flask equipped with a thermometer, reflux condenser and mechanical stirring, add 0.2mol of sodium ethylate, stir to dissolve, then add 1mol of 1,3-acetonedicarboxylic acid diethyl ester, slowly add 1mol ethyl acrylate dropwise, control the internal temperature at 25-30°C, stir at this temperature for 1h, then heat the reaction solution to 80°C, add 1.8mol sodium ethylate, stir for 4h, after the reaction is completed, concentrate and remove Absolute ethanol, the residue is the intermediate;
[0034] (2) in the 1000ml there-necked flask that thermometer, reflux condenser and mechanical stirring are housed, add the sodium hydroxide aqueous solution of 400g mass concentration 20%, then add the intermediate that step (1) makes, be heated to 70-80 ℃, After 2 hours, add concentrated hydrochloric acid dropwise to ...
Embodiment 3
[0037] A preparation method for 1,3-cyclohexanedione, comprising the steps of:
[0038] (1) Under nitrogen protection, add 250ml of anhydrous methanol to a 500ml three-necked flask equipped with a thermometer, reflux condenser and mechanical stirring, add 0.3mol of potassium carbonate, stir to dissolve, add 1mol of 1,3-acetone dicarboxylic acid dimethyl ester, slowly add 1mol methyl acrylate dropwise, control the internal temperature at 25-30°C, stir at this temperature for 1.5h, then heat the reaction solution to 70°C, add 2.7mol potassium carbonate, stir for 5h, after the reaction is complete, concentrate Remove anhydrous methanol, the residue is the intermediate;
[0039] (2) in the 1000ml there-necked flask that thermometer, reflux condenser and mechanical stirring are housed, add the sodium hydroxide aqueous solution of 400g mass concentration 10%, then add the intermediate that step (1) makes, be heated to 80-90 ℃, After 2 hours, add acetic acid dropwise to adjust the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com