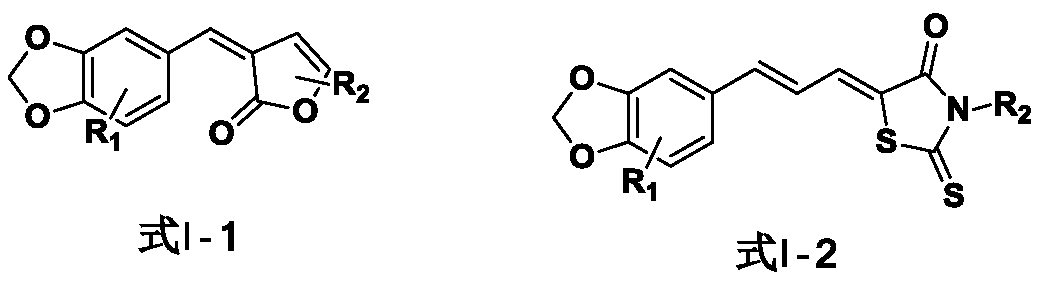
Compound with piperine skeleton structure as well as preparation and application thereof
A skeleton compound, piperine technology, applied in the field of agricultural pest control, can solve the problems of heavy workload, long time-consuming, high cost, etc., and achieve the effect of low difficulty, novel skeleton and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
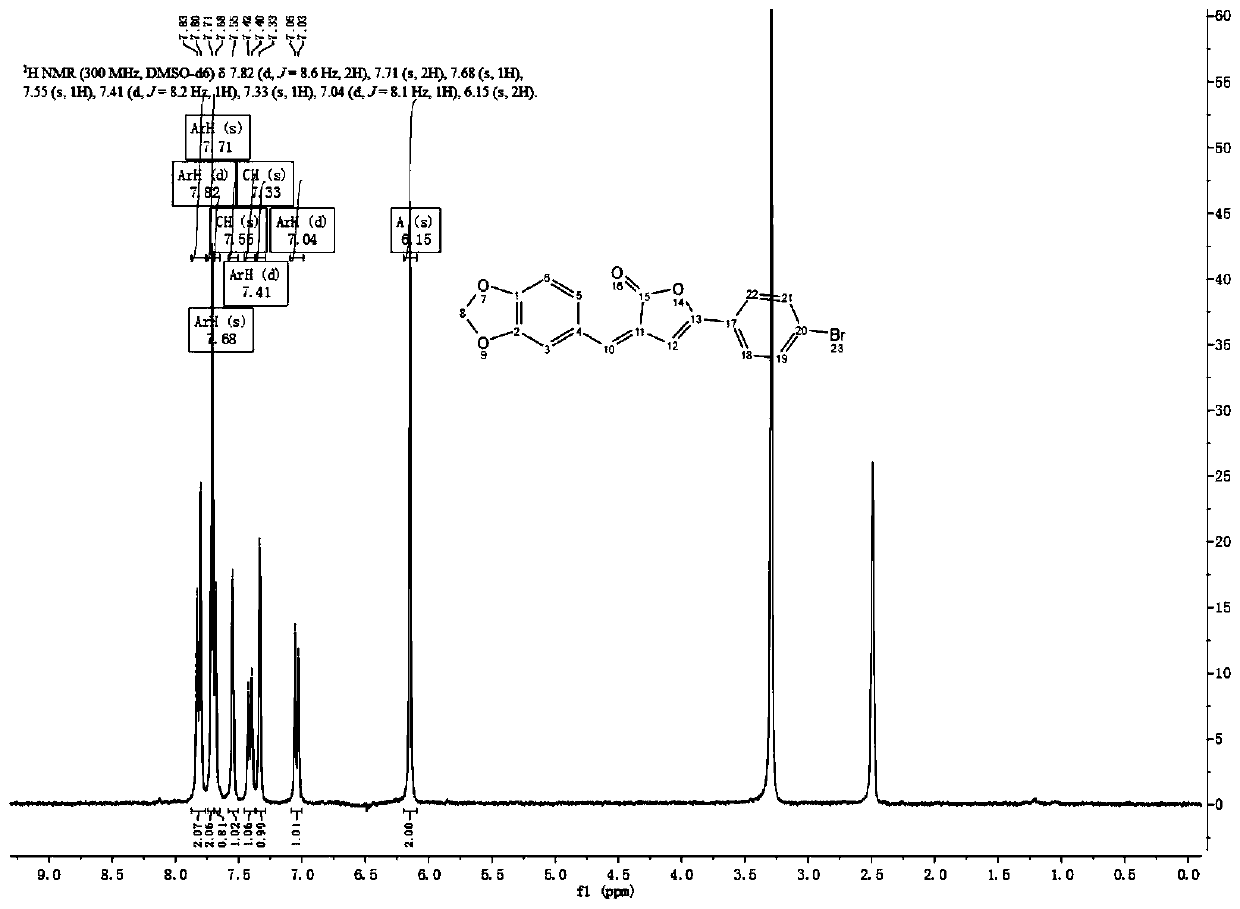
[0034] Embodiment 1: the preparation of the piperonyl compound I-1-(3) containing angelica lactone
[0035] Add raw material 1 (1.22g, 8.0mmol), DCM 50ml and manganese dioxide (6.96g, 80mmol) into a 250ml three-necked reaction flask, stir and react at room temperature for 2-5h, and monitor the completion of the reaction by TLC. Diatomaceous earth was filtered to remove manganese dioxide, and the filtrate was concentrated under reduced pressure to obtain white solid 3,4-methylenedioxybenzaldehyde 2 (1.12 g, 7.46 mmol), with a yield of 93.25%.
[0036]
[0037] Add raw material 3 (539mg, 2.1mmol), acetic anhydride (3.84g, 37.8mmol) and THF10ml into a 50ml reaction flask, stir at room temperature for 10min, add 3 drops of concentrated sulfuric acid, continue stirring for 2-3h, monitor the reaction by TLC Finish. Add an appropriate amount of water, continue to stir and react for 15-20 minutes, concentrate under reduced pressure, add an appropriate amount of methanol, and a lar...
Embodiment 2
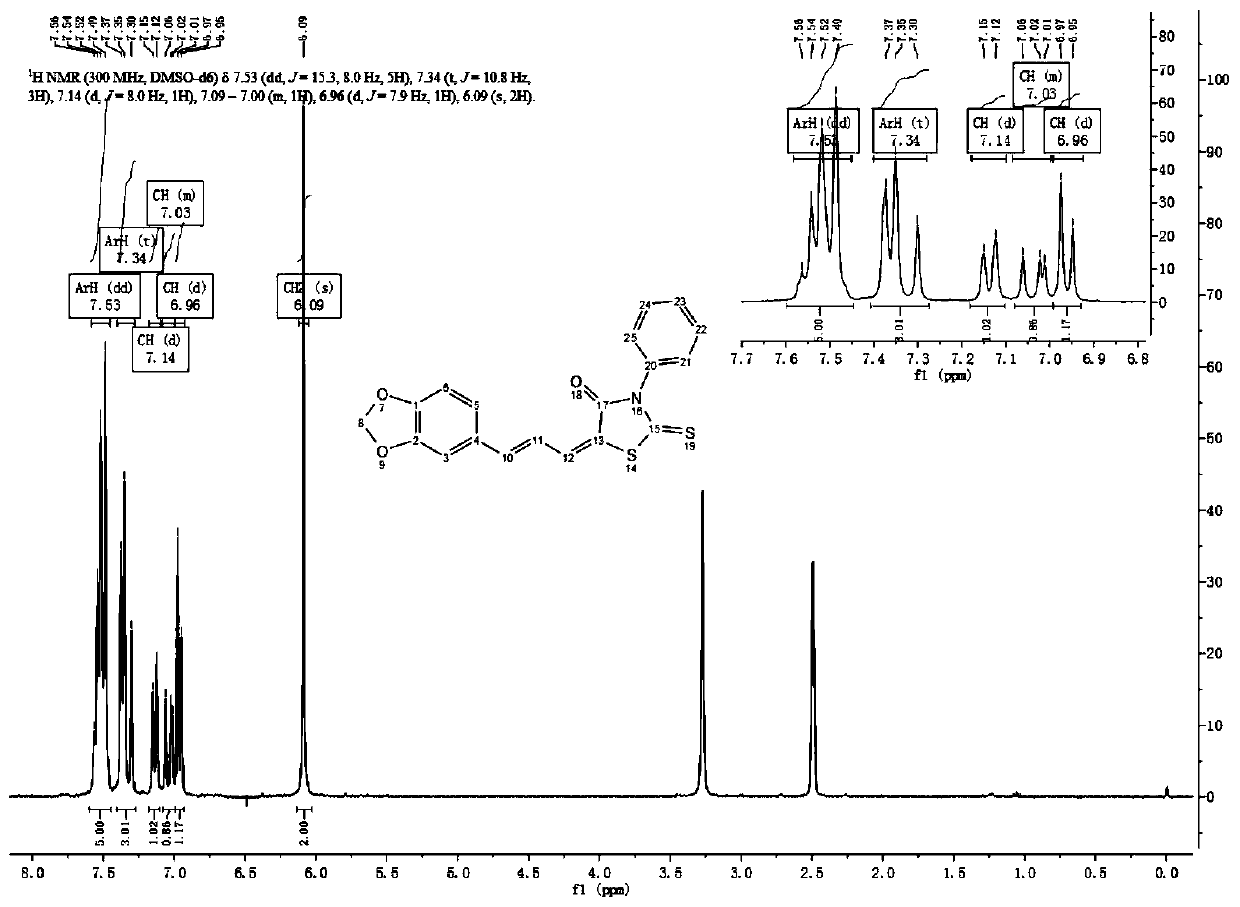
[0044] Embodiment 2: Preparation of piperonyl ring compound I-2-(10) containing thiothiazolidinone
[0045] Synthesis of 3,4-methylenedioxybenzaldehyde 2:
[0046]
[0047] Add raw material 1 (1.22g, 8.0mmol), DCM 50ml and manganese dioxide (6.96g, 80mmol) into a 250ml three-necked reaction flask, stir and react at room temperature for 2-5h, and monitor the completion of the reaction by TLC. Diatomaceous earth was filtered to remove manganese dioxide, and the filtrate was concentrated under reduced pressure to obtain white solid 3,4-methylenedioxybenzaldehyde 2 (1.12 g, 7.46 mmol), with a yield of 93.25%.
[0048] Synthesis of 3,4-methylenedioxycinnamaldehyde 6:
[0049]
[0050] Add raw material 2 (450 mg, 3 mmol), raw material 3 (916 mg, 3.01 mmol) and 20 ml of toluene into a 100 ml three-necked reaction flask, heat up to 80-100 ° C for 20-24 h. After the reaction was completed, it was cooled to room temperature, and unreacted raw material 5 was removed by filtration...
Embodiment 3
[0060] Example 3: Determination of Enzyme Inhibitory Activity of Compounds with Piperine Skeleton Structure
[0061] With MU-(GlcNAc) 2 As substrate, 20mM sodium chloride and 20mM sodium dihydrogen phosphate mixed solution are used as buffer solution, and experimental group and control group (+), control group (-) are set, and three repetitions are established in each group. Experimental group: In the standard reaction system, 2 μL of a certain concentration of compound and 88 μL of OfCht-I, OfChi-H, OfHex1, HsChit1 and buffer premix, incubated at 30°C for 10 minutes, then added 10 μL of substrate, and incubated at 30°C After 20 minutes, 100 μL of 0.5 M sodium carbonate was added to terminate the reaction, and the released MU was detected by a fluorescence detector for its absorption value A, with an excitation wavelength of 360 nm and an emission wavelength of 460 nm. Control group (+): 2 μL of DMSO was used to replace 2 μL of a certain concentration of the compound, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com