Quantum dot nucleic acid detection kit and method for simultaneously detecting 24 respiratory tract pathogens
A detection kit and quantum dot technology, applied in the field of biomedicine, can solve problems such as incomplete species of respiratory pathogens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0139] Implementation Case 1: Preparation and Use of the Kit for Quantum Dot Nucleic Acid Detection of Respiratory Pathogenic Bacteria in the Present Invention
[0140] 1. Quantum dot nucleic acid detection principle: Molecular hybridization is carried out between biotin-labeled nucleic acid amplification products and probes on the detection membrane strip, and then biotin is combined with streptavidin-coupled quantum dots to detect membrane strips. The strips are detected by a fluorescence detection instrument for the presence or absence of light signals at each site to determine whether the probe hybridizes with the nucleic acid product, thereby determining whether the sample contains the relevant target nucleic acid.
[0141] The capture probe is labeled with an amino group at the 3' end or 5' end of the oligonucleotide single-stranded DNA, and there is an intermediate arm between the amino group and the oligonucleotide single-stranded DNA, and the intermediate arm is a fatt...
Embodiment 2
[0273] The present invention is used for the sensitivity verification of 24 kinds of respiratory pathogenic bacteria quantum dot nucleic acid detection kits:
[0274] According to the reaction system in Example 1, 21 ul were respectively dispensed, and 4 ul of DNA / RNA was added to each reaction system, with a concentration of 5000 copies / ml and 500 copies / ml.
[0275] RT-PCR amplification program carries out PCR amplification according to the program described in embodiment 1, specifically as follows:
[0276]
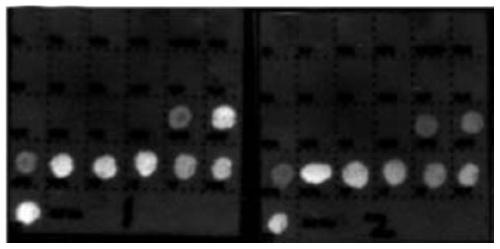
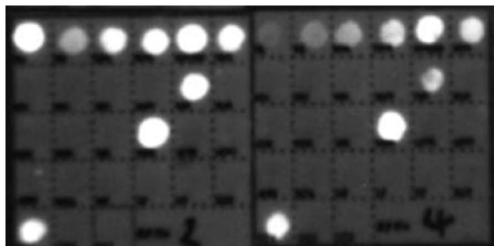
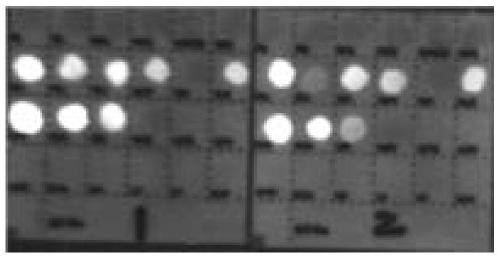
[0277] The detection process of the quantum dot gene chip is carried out according to the kit use process in Example 1 for relevant detection. The test results of the three-tube reaction can be found in figure 1 , figure 2 , image 3 . The results of fluorescence detection show that the detection target sensitivity of the kit can reach 500copies / ml.
Embodiment example 3
[0278] Implementation Case 3: Throat swab clinical sample detection
[0279] Collect 12 cases of throat swab samples suspected of respiratory tract infection according to clinical microbiology sample collection specifications, and use the virus nucleic acid extraction kit produced by Hangzhou Qianji Biotechnology Co., Ltd. to extract nucleic acid, and then use the reaction system in Example 1 to perform nucleic acid extraction Sample detection, specific amplification process and detection process refer to Example 1. The specific test results are as follows:
[0280] sample number Example test results Sequencing results 1 ND ND 2 H1N1 (2009) H1N1 (2009) 3 H1N1 (2009) H1N1 (2009) 4 229E 229E 5 HKU1 HKU1 6 ND ND 7 ND ND 8 RSVB RSVB 9 H1N1 (2009) H1N1 (2009) 10 RSVA / HKU1 RSVA / HKU1 11 H1N1 (2009) H1N1 (2009) 12 ND ND
[0281] Note: ND is marked as the detection of releva...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com