Synthetic method of isotope-labeled erythromycylamine
A technology of isotope labeling and synthesis method, which is applied in the field of drug synthesis and achieves the effects of reasonable process design, strong operability and short synthesis method route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
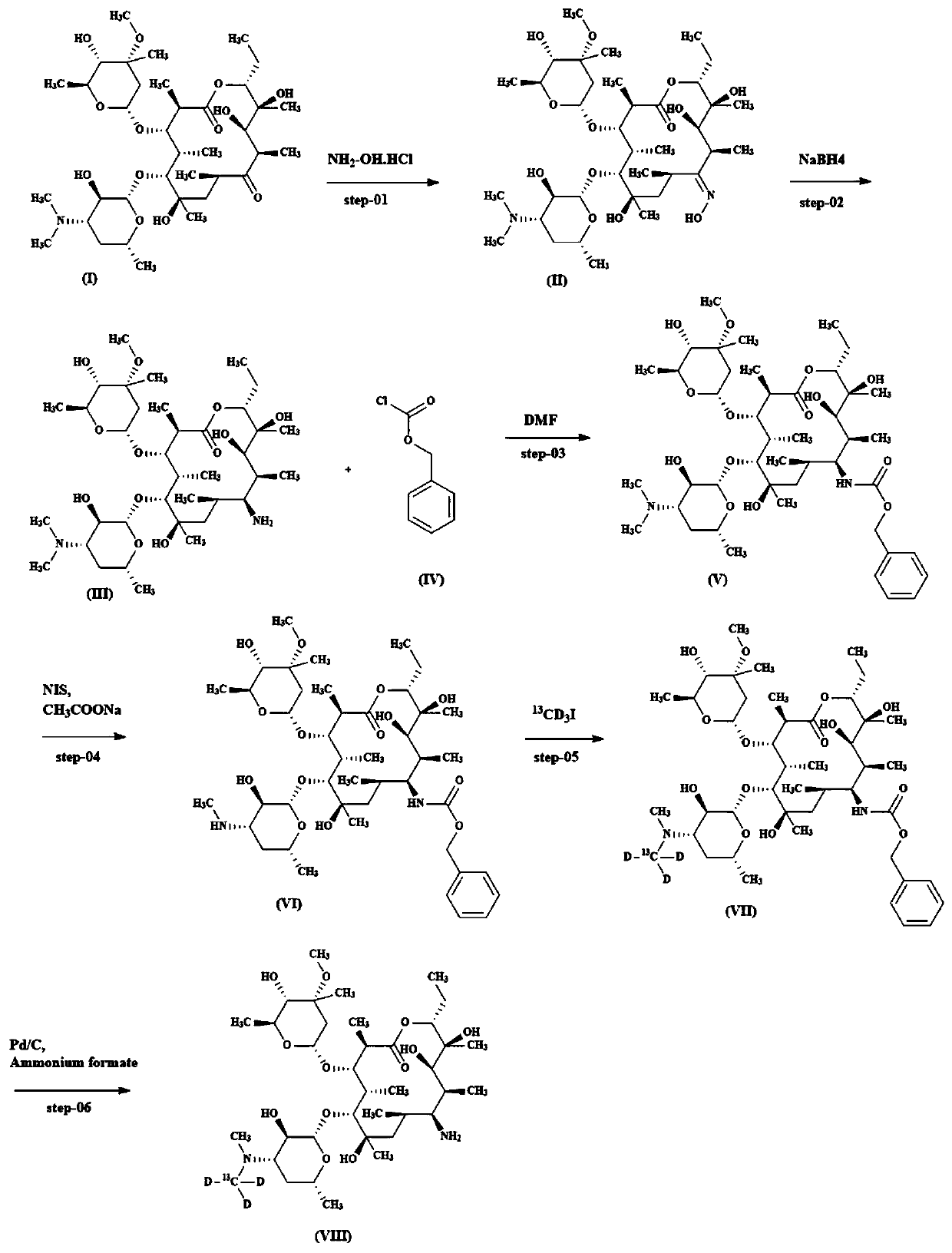
[0026] Such as figure 1 Shown, a kind of synthetic method of isotope-labeled erythromycylamine comprises the following steps:
[0027] (1) Get 20g of erythromycin, add it to a 150mL round-bottomed flask, dissolve it with 50mL of methanol, then add 10g of hydroxylamine hydrochloride and 8g of potassium carbonate successively, and react for 48 hours at 20°C to obtain a white suspension; filter and spin the filtrate Dry, then add 100mL of water, adjust the pH to 10 with 25% ammonia water under ice bath conditions, a white solid precipitates out, filter to obtain 18g of intermediate II, which is a white solid, and the yield is 88.20%;
[0028] (2) Dissolve 18g of intermediate II in 90mL of methanol, slowly add 3g of sodium borohydride under ice bath conditions, and react for 8 hours at 20°C. The TLC board monitors that the raw materials have reacted completely. Add 300mL of water to the reaction solution, and use two Chloromethane extraction, the dichloromethane phase was separat...
Embodiment 2
[0034] Such as figure 1 Shown, a kind of synthetic method of isotope-labeled erythromycylamine comprises the following steps:
[0035] (1) Take 20g of erythromycin, add it to a 150mL round-bottomed flask, dissolve it with 50mL of methanol, then add 10g of hydroxylamine hydrochloride and 13g of triethylamine in sequence, and react at 20°C for 48 hours to obtain a white suspension; filter and filtrate Spin dry, then add 100mL of water, adjust the pH to 10 with 25% ammonia water under ice bath conditions, a white solid precipitates out, filter to obtain 12g of intermediate II, which is a white solid, and the yield is 55.80%;
[0036](2) Dissolve 12g of intermediate II in 120mL of methanol, slowly add 6g of lithium borohydride under ice bath conditions, react at 40°C for 8 hours, monitor the complete reaction of the raw materials on a TLC board, add 300mL of water to the reaction solution, and use two Chloromethane extraction, the dichloromethane phase was separated, concentrated...
Embodiment 3
[0042] Such as figure 1 Shown, a kind of synthetic method of isotope-labeled erythromycylamine comprises the following steps:
[0043] (1) Get 20g of erythromycin, add it to a 150mL round-bottomed flask, dissolve it with 50mL of ethanol, then add 10g of hydroxylamine hydrochloride, 8g of potassium carbonate, and react at 40°C for 12 hours to obtain a white suspension; filter and spin the filtrate Dry, then add 100mL of water, adjust the pH to 10 with 25% ammonia water under ice bath conditions, a white solid precipitates out, filter to obtain 14g of intermediate II, which is a white solid, and the yield is 68.60%;
[0044] (2) Dissolve 14g of intermediate II in 90mL of methanol, slowly add 3g of lithium borohydride under ice bath conditions, and react for 8 hours at 20°C. The TLC board monitors the complete reaction of the raw materials. Add 300mL of water to the reaction solution, and use two Chloromethane extraction, the dichloromethane phase was separated, concentrated, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com